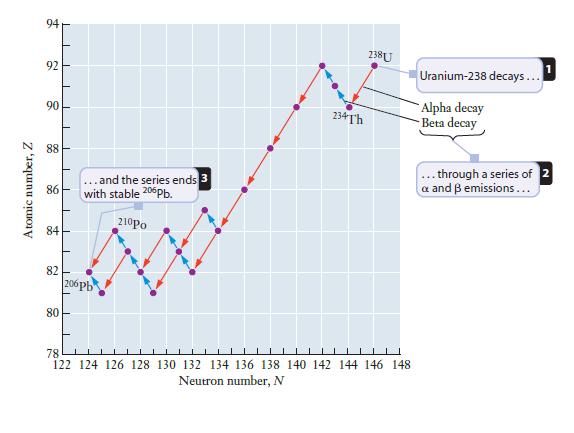
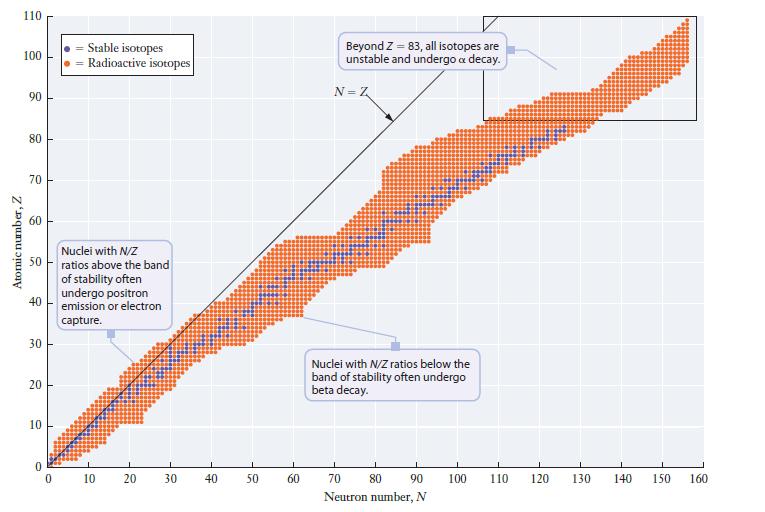
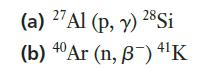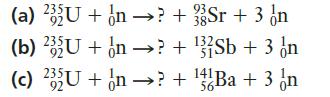Chemistry For Engineering Students 4th Edition Lawrence S. Brown, Tom Holme - Solutions
Unlock the potential of "Chemistry For Engineering Students 4th Edition" by Lawrence S. Brown and Tom Holme with our comprehensive collection of resources. Access an extensive range of online solutions, including a detailed answers key and solutions manual, ensuring you have the answers you need. Our Solutions PDF features solved problems and questions and answers, providing a robust test bank and chapter solutions for thorough understanding. Benefit from step-by-step answers found in the instructor manual, enhancing your grasp of the textbook content. Explore the convenience of free download options for an enriched learning experience.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()