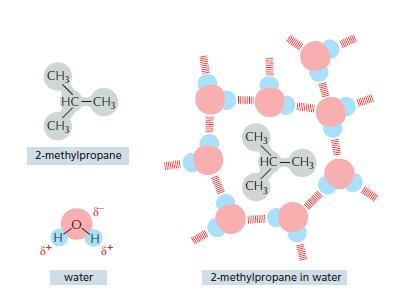
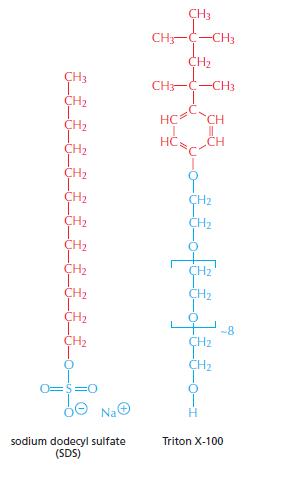
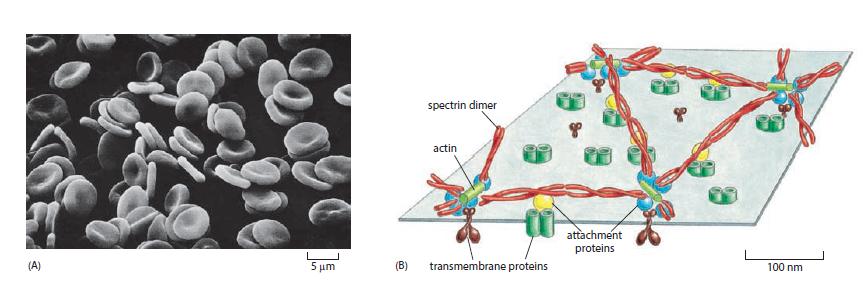
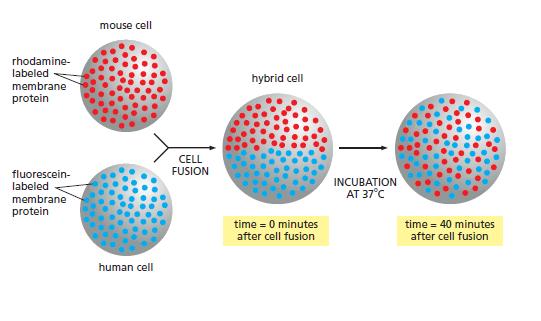
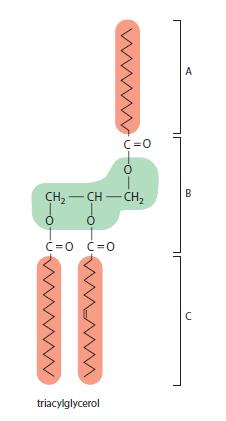
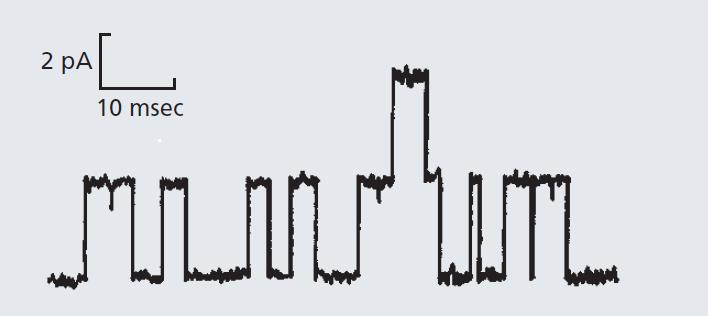
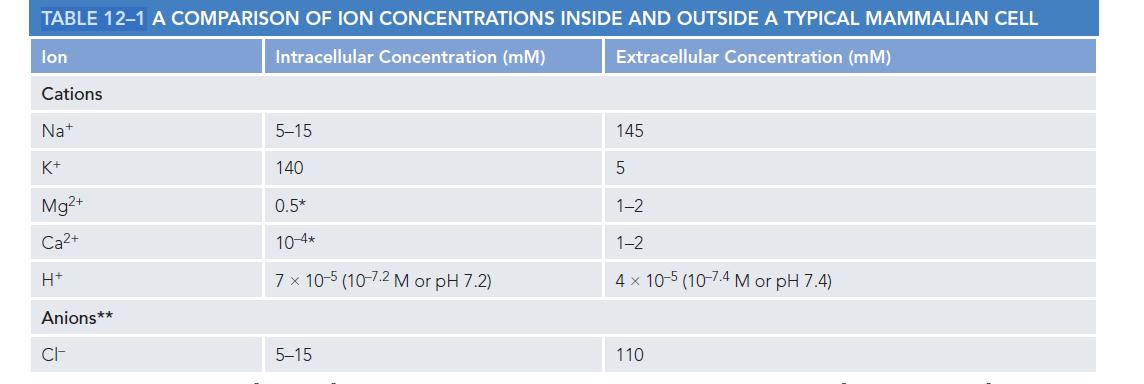
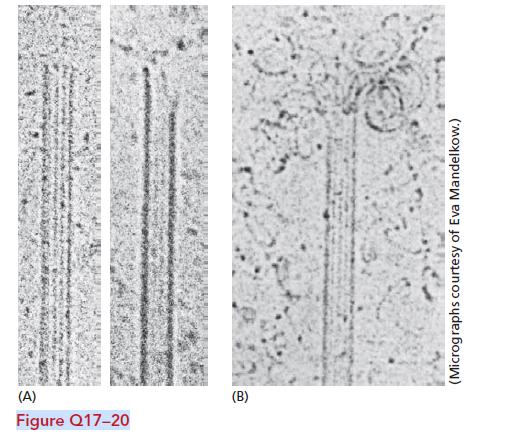
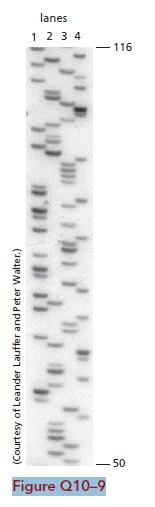
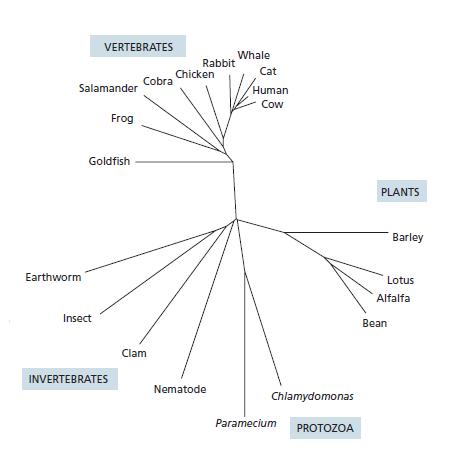
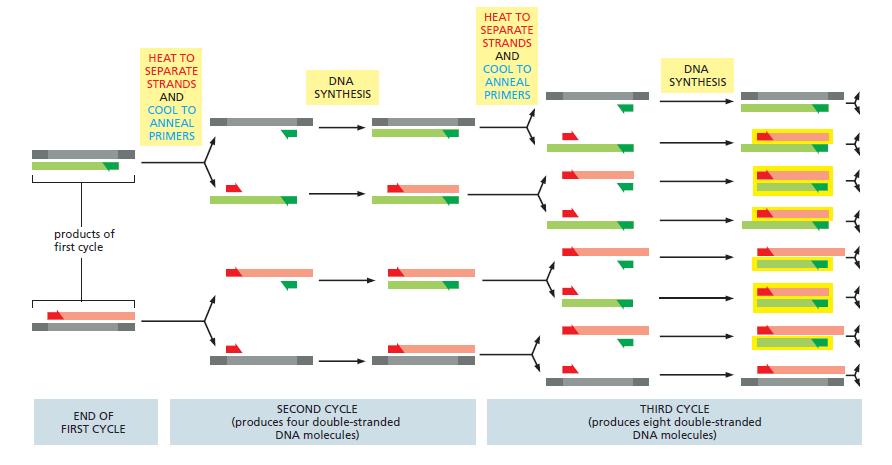
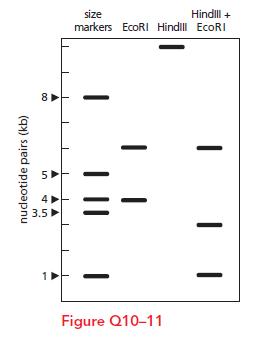
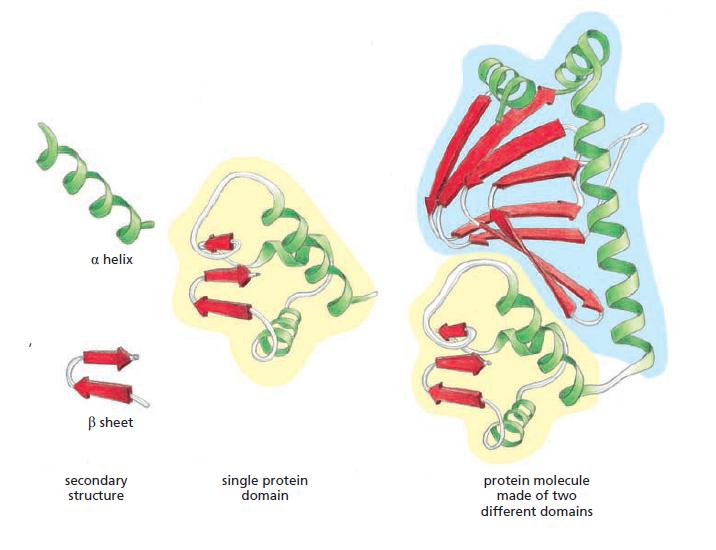
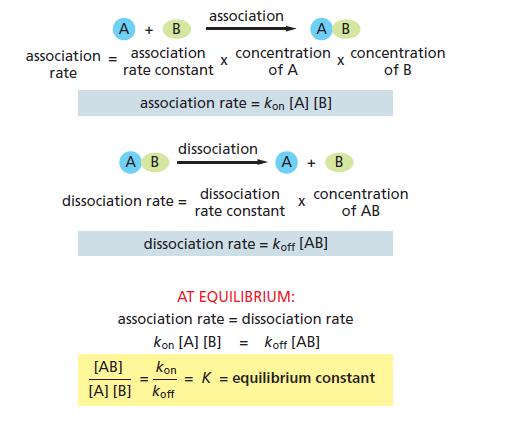
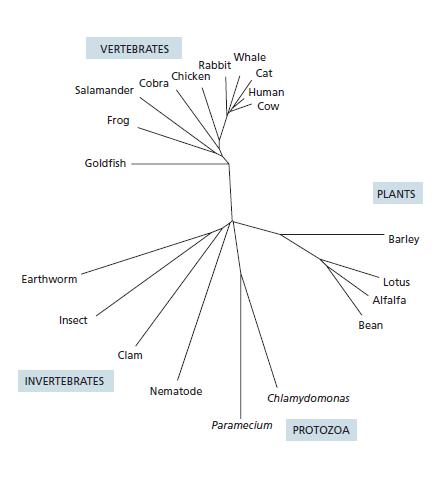
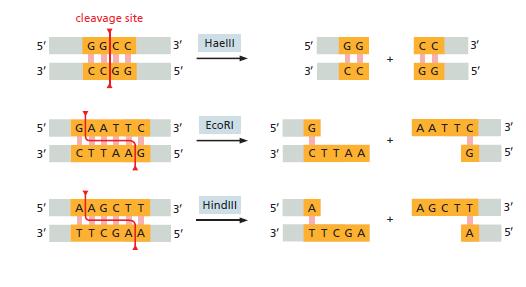
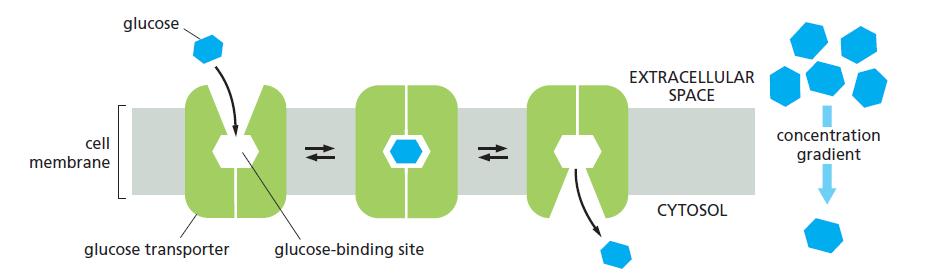
Essential Cell Biology 5th Edition Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter - Solutions
Unlock the full potential of "Essential Cell Biology 5th Edition" with our comprehensive online solutions. Access an extensive answers key and solution manual to enhance your understanding of complex biological concepts. Our solutions PDF offers step-by-step answers and solved problems, perfect for mastering your textbook content. Delve into our test bank for chapter solutions that provide clear insights and detailed explanations. Whether you're a student or an instructor, our manual ensures you have the tools needed for success. Enjoy the convenience of free download options for seamless learning and exploration.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()