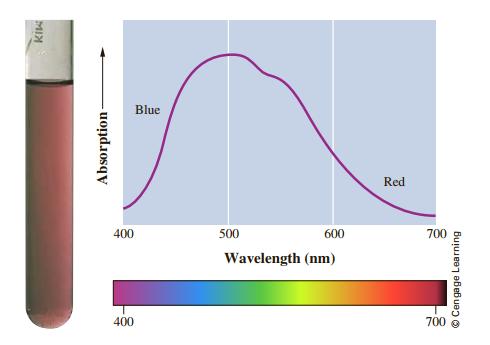
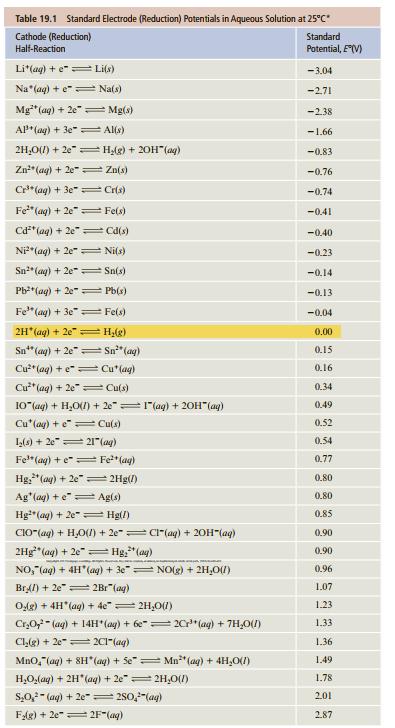
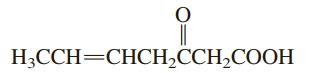General Chemistry 11th Edition Darrell Ebbing, Steven D. Gammon - Solutions
Unlock the secrets of mastering chemistry with our comprehensive resource for "General Chemistry 11th Edition" by Darrell Ebbing and Steven D. Gammon. Access a treasure trove of online answers key, solutions manual, and solutions in PDF format, all tailored to enhance your understanding. Dive into solved problems and explore detailed questions and answers designed to prepare you for success. Our test bank and chapter solutions offer step-by-step answers, ensuring clarity and confidence. Whether you're using the instructor manual for teaching or the textbook for learning, enjoy the convenience of free download options to support your educational journey.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()