Assuming that oxide-ion conductivity of YBaCu3O7-8 at 500 C in air is (vo) = 10-S/m and the
Question:
Assuming that oxide-ion conductivity of
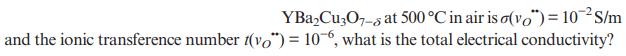
Transcribed Image Text:
YBaCu3O7-8 at 500 C in air is (vo") = 10-S/m and the ionic transference number (vo") = 106, what is the total electrical conductivity?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The ionicconductivity tr...View the full answer

Answered By

PU Student
cost accounting
financial accounting
auditing
internal control
business analyst
tax
i have 3 years experience in field of management & auditing in different multinational firms. i also have 16 months experience as an accountant in different international firms. secondary school certification.
higher secondary school certification.
bachelors in mathematics.
cost & management accountant
4.80+
4+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
A nanolaminated material is fabricated with an atomic layer deposition process, resulting in a series of stacked, alternating layers of tungsten and aluminum oxide, each layer being = 0.5 nm thick....
-
Conductivity and contactless conductivity detectors were developed for suppressed capillary ion chromatography. Observed peak heights in millivolts for bromide standards are in the table. a. Use...
-
When nickel(II) oxide is heated in O 2 , some of the cations are oxidized and vacant cation sites are formed according to the equation: where h + denotes a vacant cation site and (s) denotes an ion...
-
Laverty Clinic plans to purchase a new centrifuge machine for its New York facility. The machine costs $94,000 and is expected to have a useful life of 6 years, with a terminal disposal value of...
-
Knowing that the shaded area is equal to 6000 mm2 and that its moment of inertia with respect to AA² is 18 Ã 106 mm4, determine its moment of inertia with respect to BB² for d1 = 50...
-
Government involvement in litigation is mainly to provide a set of procedural rules, court facilities, and judges. Should government be expected to provide whatever resources are needed to deal...
-
Use Tables V, VI, VII, and VIII in Appendix D to find each of the following F-values: a. F.05, v1 = 4, v2 = 4 b. F.01, v1 = 4, v2 = 4 c. F.10, v1 = 30, v2 = 40 d. F.025, v1 = 15, v2 = 12
-
A plane wall of a furnace is fabricated from plain carbon steel (k = 60 W/m K, p = 7850 kg/m 3 , c = 430 J/kg K) and is of thickness L = 10 mm. To protect it from the corrosive effects of the...
-
Sanford, Inc., has developed value - added standards for four activities: purchasing parts, receiving parts, moving parts, and setting up equipment. The activities, the activity drivers, the standard...
-
Calculate the enhancement factor D O /D h for Equation (3.34). Equation (3.34) Do 1 + D 4c Dr. Ch 4cv Ch
-
A rectangle of calcium-stabilized zirconia Zro.85 Cao.150 1.85 (a = 5.13 ) is covered with porous platinum electrodes on its opposite faces, heated to 1100 C in air, and an electrical conductivity of...
-
Recognizing the importance of planning for their future, Sean and Karen sat down to determine their net worth. They compiled a list of the assets and liabilities each one of them is bringing into the...
-
Machine-hours required to support estimated production Fixed manufacturing overhead cost Variable manufacturing overhead cost per machine-hour Required: 1. Compute the plantwide predetermined...
-
Assume now that a new firm (firm N) discovers and patents a more efficient technology, summarized by thetotal cost function C = 10q. The new technology can be used only by the new firm, which enters...
-
1. How has Dell used virtual integration to become an industry leader? Dell has used virtual integration to become an industry leader by leveraging its global suppliers to reduce costs and provide...
-
3) Consider the asset pricing model with uncertainty in the slide. We derived the asset prices as Pb = Ps = - [nu' (y+Yn + e) + (1 )u' (y + y + e)] u'(e1) [nu' (y +n + ) + (1 )u' (y + y + e2)] u'(e1)...
-
Amazon is considered a leader in managing its supply chain. Describe in detail two parts of Amazon's Supply Chain Management that you see as critical to their success. Please provide your reasoning...
-
When 200 convicted embezzlers were randomly selected, the mean length of prison sentence was found to be 22.1 months and the standard deviation was found to be 8.6 months. Kim Patterson is running...
-
What is the purpose of the journal wizard?
-
How many signals do you expect in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) Geraniol Isolated from roses and used in perfumes . NH2 Dopamine A neurotransmitter that is...
-
Rank the signals of the following compound in terms of increasing chemical shift. Identify the proton(s) giving rise to each signal: CI. .
-
Predict the expected number of signals in the 13 C NMR spectrum of each of the following compounds. For each signal, identify where you expect it to appear in the 13 C NMR spectrum: (a) (b) (c)
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...

Study smarter with the SolutionInn App


