Question: Consider these six-coordinate ionic radii for divalent, first-row transition-metal ions: r(Ti+) = 0.86 , r(V+) = 0.79 , r(Cr+) = 0.80 , r(Mn+) = 0.83
Consider these six-coordinate ionic radii for divalent, first-row transition-metal ions:
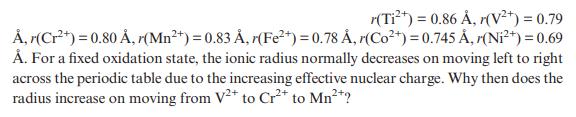
r(Ti+) = 0.86 , r(V+) = 0.79 , r(Cr+) = 0.80 , r(Mn+) = 0.83 , r(Fe+) = 0.78 , r(Co+) = 0.745 , (Ni+) = 0.69 . For a fixed oxidation state, the ionic radius normally decreases on moving left to right across the periodic table due to the increasing effective nuclear charge. Why then does the radius increase on moving from V2+ to Cr+ to Mn+?
Step by Step Solution
3.46 Rating (162 Votes )
There are 3 Steps involved in it
The deviation from the general trend is due to occupation ... View full answer

Get step-by-step solutions from verified subject matter experts


