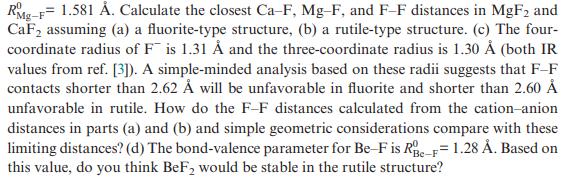
Question: The bond-valence parameters for C aF and MgF bonds are R 0 C aF = 1.842 and
The bond-valence parameters for Ca–F and Mg–F bonds are R0 CaF= 1.842 Å and
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Equation 518 a In fluorite the cation is 8coordinated and the ideal MF bond valence is 28 14 Pluggin... View full answer

Get step-by-step solutions from verified subject matter experts


