The images below show a portion of a chain of the conducting polymer polypyrrole, before (top) and
Question:
The images below show a portion of a chain of the conducting polymer polypyrrole, before (top) and after (bottom) oxidative doping.
(a) Is the charge carrier formed in this process a polaron, bipolaron, or soliton?
(b) Is the conducing polymer that results doped n- or p-type?
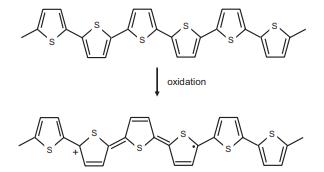
Transcribed Image Text:
oxidation S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a A single carbocationradical ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Company History. Wawa Wild Wings, (WWW) was formed in 1980 by 3 residents of the town of Wawa Ontario, located about 230 kilometers north of Sault Ste. Marie Ontario. The 3 equal partners are Cosmo...
-
The September 30, 2018, adjusted trial balance of Buzzy?s, Inc., is shown next. Requirements 1. Prepare the September closing entries for Buzzy?s, Inc. 2. Calculate the ending balance in Retained...
-
The mass of 0 can be measured by observing the reaction + p 0 + n at very low incident kinetic energy (assume it is zero). The neutron is observed to be emitted with a kinetic energy of 0.60MeV....
-
A strip bond that will mature 7_1 years from now at its $13,000 face value can be 2 purchased today for $9042. What rate of return (compounded semiannually) will this strip bond provide to an...
-
Suppose an African American couple opens a restaurant that serves African cuisine, hoping to appeal mostly to people of African descent. The restaurant is a big success, yet the couple finds that...
-
Identify common inherent risk factors that companies involved in the entertainment industry pose for their independent auditors. List and briefly describe specific audit procedures that would not be...
-
ork /Test Score: 0 of 20 pts 2 of 8 (6 complete) HW Score: 65%, 65 of 100 pts E13-23 (similar to) Question Help The charter for KCAS - TV, Inc. authorizes the company to issue 100,000 shares of $7....
-
Consider the electronic structure of graphene. (a) At how many points in the first Brillouin zone do the conduction and valence bands of graphene meet? (b) What is the name given to those points? (c)...
-
Each of the following compounds contains an octahedrally coordinated transition metal ion and behaves as an insulator/semiconductor. Identify each as either a band insulator or a MottHubbard...
-
An automobile suspension system has three physical state variables, as shown in Figure AP11.5. The state variable feedback structure is shown in the figure, with K 1 = 1 Select K 2 and K 3 so that...
-
The current rate of interest on S-T Treasury Bills = 10%, intermediate term Gov. Bonds = 11%, Lt- Gov. Bonds = 12%, AA rate Corp. Bonds = 13.5% and the rate of inflation is 5%. Holding-period returns...
-
Prepare Income Statement(absorption costing) for the second, third and fourth month. SALES (SP X unit sold) INCOME STATEMENT FORMAT (ABSORPTION COSTING) XXX Less: Cost of Goodsold VARIABLE COST (VC...
-
The following shows the distribution of final exam scores in a large introductory psychology class. The proportion under the curve is given for two segments (short answers-no calculations required)....
-
How much overhead was included in the cost of Job #461 at the beginning of January? * (1 Point). BREAD Co. uses a job order costing system. At the beginning of January, the company had 2 jobs in...
-
3. (3pt.) A state of a physical system is just a description of the system at an instant in time in terms of its properties. In classical mechanics, states are represented by points (in phase space)....
-
Referring back to Chapter 2, explain why the official data for women's labor force participation in 1890 given in Table 5-1 provide a misleading impression of the relationship between women's labor...
-
a) Calculate the goodwill that was paid by Major Ltd on the acquisition of Minor Ltd. [10 marks] b) Prepare the consolidated statement of financial position for Major Ltd at 31 July 20X8. [30 marks]...
-
From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data Tables), calculate the standard enthalpy of formation of H 2 S(g) and of FeS 2 (s): AR(kJ mol) Fe(s) + 2H2S(g) ...
-
Which of Ne or Ar has the larger van der Waals parameter b? Explain your reasoning.
-
You have calculated the pressure exerted by ethane using the ideal gas law and the RedlichKwong equations of state. How do you decide if the repulsive or attractive part of the molecular potential...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...
-
Horizontal Analysis The comparative accounts payable and long-term debt balances of a company are provided below. Current Year Previous Year Accounts payable $47,286 $63,900 Long-term debt 85,492...
-
On January 1, Year 1, Price Company issued $140,000 of five-year, 7 percent bonds at 97. Interest is payable annually on December 31. The discount is amortized using the straight-line method. Record...

Study smarter with the SolutionInn App


