EXERCISE 10.7. The cyclic set of chemical reactions provides a textbook example of the use of Onsager's
Question:
EXERCISE 10.7. The cyclic set of chemical reactions
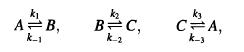
provides a "textbook" example of the use of Onsager's relations. When the reactions are out of equilibrium they produce an amount of entropy/volume time, o, where To JA +J2A2 + J3A3, Ja = (1/V)(da/dt) is the current for the ath reaction, and Aa is the affinity for the ath reaction. Assume that the chemical potential is that of an ideal gas mixture of molecules, A, B, and C.
(a) Show that near equilibrium, J - J3 = L11A1L12A2 and find L11, L12, L21, L22 and J2 J3L21A1 + L22A2.
(b) Show that L12 L21.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:






