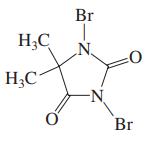
(a) The compound shown in the margin, with the common name 1,3-dibromo-5,5-dimethylhydantoin, is useful as a source...
Question:
(a) The compound shown in the margin, with the common name 1,3-dibromo-5,5-dimethylhydantoin, is useful as a source of electrophilic bromine (Br+) for addition reactions. Give a more systematic name for this heterocyclic compound.
(b) An even more remarkable heterocyclic compound (ii) is prepared by the following reaction sequence. Using the given information, deduce structures for compounds i and ii, and name the latter.
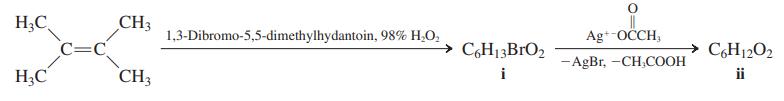
Heterocycle ii is a yellow, crystalline, sweet-smelling compound that decomposes upon gentle heating into two molecules of acetone, one of which is formed directly in its n→ π* excited state. This electronically excited product is chemiluminescent.
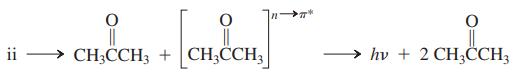
Heterocycles similar to compound ii are responsible for the chemiluminescence produced by a number of species [e.g., fireflies and several deep-sea fish]; they also serve as the energy sources in commercial chemiluminescent products.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore




