Treatment of the following epoxide with aqueous acid produces a carbocation intermediate that reacts with water to
Question:
Treatment of the following epoxide with aqueous acid produces a carbocation intermediate that reacts with water to give a diol product. Show the structure of the carbocation, and propose a mechanism for the second step.
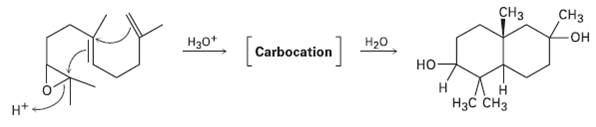
Transcribed Image Text:
ҫНз CHз [c Hзо* он Carbocation son] Нао но- Н Нас снз H*
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (21 reviews)
12 0 CH3 H3 C CH3 prot...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Refer to Figure and propose a mechanism for the final step in the Edman degradationthe acid-catalyzed rearrangement of the ATZ derivative to the PTH derivative.
-
Explain which of the following epoxide products is formed when the chlorohydrins reactant is treated with base.
-
Base treatment of the following ?, ?-unsaturated carbonyl compound yields an anion by removal of H from the y carbon. Why is hydrogen?s on the y carbon atom acidic? LDA 1:0-I I.
-
The control features of a bank account do not include: (a) having bank auditors verify the correctness of the bank balance per books. (b) minimizing the amount of cash that must be kept on hand. (c)...
-
Which factors complicate environmental analysis at the global level? Which factors are making such analysis easier?
-
With organizational cost-cutting the world over, orientation for new staff has been cut short to just several days. How will this affect retention?
-
2. Interest income that should appear in the 2016 consolidated income statement for Pangs bond issue is: a $14,000 b $10,400 c $10,000 d None of the above
-
Assume the same information as in E14-4B, except that McGee Company uses the effective-interest method of amortization for bond premium or discount. Assume an effective yield of 6% in pricing the...
-
Explain why newspapers refer to the fact that the published statistics are audited. (6 Marks) 1.2. State whether the fact that the figures are audited means that the audit body is certifying that the...
-
You want to park your bicycle in a bicycle parking area where bike racks are aligned in a row. There are already N bikes parked there (each bike is attached to exactly one rack, but a rack can have...
-
What products would you expect to obtain from reaction of 1-methylcyclo-hexanol with the following reagents? (a) HBr (b) NaH (c) H2SO4 (d) Na2Cr2O7
-
Benzoquinone is an excellent dienophile in the DielsAlder reaction. What product would you expect from reaction of Benzoquinone with 1 equivalent of 1, 3-butadiene from reaction with 2 equivalents of...
-
One classic application of correlation involves the association between the temperature and the number of times a cricket chirps in a minute. Listed below are the numbers of chirps in 1 min and the...
-
Peninsula Community Health Services of Alaska had just completed of a merger of two organizations. The original Peninsula Community Health center was a community health center only, but the CHC had...
-
Compensation Approach: Imagine that the HR department of your chosen organization from below is going to design a compensation approach for the job that is aligned with reinforcement, expectancy, and...
-
A boat leaves port and follows a course of N77E at 9 knots for 3 hr and 20 min. Then, the boat changes to a new course of S26E at 12 knots for 5 hr. Part 1 of 3 (a) How far is the boat from port?...
-
The aggregate supply curve of an economy is depicted by AS, shown in the graph on the right. Suppose that labour unions grant concessions, enabling firms to pay lower wages to their workers. Use the...
-
what is Medibank pestle analysis in term of these 2 statements? Current problem at hand deviates towards the fact that customers do not have high awareness of the health and wellbeing programs that...
-
Describe the role and responsibilities of the board of directors in corporate governance AppendixLO1
-
1. Advertising for eyeglasses _________ (increases/decreases) the price of eyeglasses because advertising promotes _________. 2. An advertisement that succeeds in getting consumers to try the product...
-
A certain mineral has a cubic unit cell with calcium at each corner, oxygen at the center of each face, and titanium at its body center. What is the formula of the mineral? An alternate way of...
-
(a) An aqueous solution of pure stereoisomer X of concentration 0.10 g mL-1 had an observed rotation of -300 in a 1.0-dm tube at 589.6 nm (the sodium D line) and 25oC. What do you calculate its [a]D...
-
Unknown Y has a molecular formula of C3H6O2. It contains one functional group that absorbs infrared radiation in the 3200-3550-cm-1 region (when studied as a pure liquid; i.e., "neat"), and it has no...
-
Which atoms in each of the following molecules are chirality centers? (a) (b) (c) (d) OH OH Lactic acid Glyceraldehyde OH HO 0 0 OH Ascorbic acid (vitamin C) OH HO Estradiol (an estrogen)
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


