Explain the fact that, although hemiacetal formation between methanol and cyclohexanone is thermodynamically disfavored, addition of methanol
Question:
Explain the fact that, although hemiacetal formation between methanol and cyclohexanone is thermodynamically disfavored, addition of methanol to cyclopropanone goes essentially to completion:
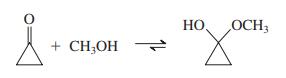
Transcribed Image Text:
HO НО OCH3 + CH;OH 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Hemiacetal formation bw methanol and cyclohexanone is thermodynamically unfavorable This can be ...View the full answer

Answered By

Bharat sharma
• Strong theoretical and practical knowledge of chemistry
• In-depth knowledge of chemistry and ability to teach students
• Ability to develop and execute lessons plans
• Skilled in a variety style of teaching methods and techniques
• Experience of conducting research and documenting results
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
How can you explain the fact that franc-i -hromo-2-methylcyclohcxane yields the non-Zaitsev?s elimination product 3-methylcyclohexene on treatment with base? H C Br trans-1-Bromo-2-methylcyclohexane...
-
How might Kekule explain the fact that there is only one dibromobenzene with the bromines on adjacent carbon atoms, even though we can draw two different structures, with either a double or a single...
-
The Expectations Theory of the Term Structure cannot explain the fact that treasury yield curves are generally upward sloping, and become downward sloping only infrequently. How can the Liquidity...
-
Match the following ratios with the appropriate formula. Ratio or Rate Formula a. Income from operations Interest expense Acid-test Total liabilities Stockholders' equity Current b. Net income-...
-
Consider the two-period com economy of the text. Suppose that initially the equilibrium has a zero trade deficit. Now, increase the interest rate. Show how the Fisher diagram changes. Will trade...
-
True or False: Applets cannot create files on the users system.
-
Kellogg Corporation is the worlds leading producer of ready-to-eat cereal products. In recent years the company has taken numerous steps aimed at improving its profitability and earnings per share....
-
A partial summary of the payroll data for Burrington Manufacturing Company for each week of June is as follows: a. Compute the missing amounts in the summary, assuming that no employees have reached...
-
Urgent Help with accounting problem!! Your portfolio project will provide specific answers to the questions that follow. Apply what you have learned in this course to your answers to these questions....
-
WAR (We Are Rich) has been in business since 1987. WAR is an accrual-method sole proprietorship that deals in the manufacturing and wholesaling of various types of golf equipment. Hack & Hack CPAs...
-
Propose efficient syntheses of each of the following molecules, beginning with the indicated starting materials. (a) (b) (c) from H;C HO, H
-
The rate of the reaction of NH2OH with aldehydes and ketones is very sensitive to pH. It is very low in solutions more acidic than pH 2 or more basic than pH 7. It is highest in moderately acidic...
-
For each of the following situations: a. Discuss the key issues to address in determining whether or not revenue should be recognized. b. Identify additional information required or audit procedures...
-
FACTS: The Budvar Company sells parts to a foreign customer on December 1, Year 1, with payment of 20,000 crowns to be received on March 1, Year 2. Budvar enters into a forward contract (with a...
-
A production Edgeworth Box, with origins indicated for the inputs of capital, K , and labor, L , into production of goods X and Y .Eight isoquants are shown, reflecting standard...
-
For 2014, Nichols, Inc., had sales of 150,000 units and production of 200,000 units. Other information for the year included: Direct manufacturing labor 187,500 Variable manufacturing overhead...
-
reading the following statement and decide whether you agree or disagree with the statement: "The free market system is the best economic system since it is the most efficient and solves basic...
-
find the net presbf value of the project ? present value index? Net present value A project has estimated annual net cash flows of $11,250 for 10 years and is estimated to cost $42,500. Assume a...
-
Rewrite Example 13.51 in Java.
-
Explain how two samples can have the same mean but different standard deviations. Draw a bar graph that shows the two samples, their means an standard deviations as error bars. T S
-
What is the hybridization at the N and each C in this molecule? Indicate the type of bond and the orbitals that are overlapping to form it for each of the designated bonds (for example, ?CSP3 + H1s)....
-
Consider hydrogen cyanide, H C N. (a) What is the hybridization at the N at the C? (b) What are the types of the three CN bonds? What orbitals overlapping to form them? (c) In what type of orbital...
-
What is the hybridization at each C in this molecule? Indicate the type of bond and the orbital's that are overlapping to form it for each of the designated bonds? ITT H=C=C=C=C_C7H (both) H tall...
-
You purchased a stock at a price of $38.68. The stock paid a dividend of $1.18 per share. What was the dividend yield? Convert to a percentage and round to one place past the decimal point.
-
Determining missing items in return and residual income computations Data are presented in the following table of returns on investment and residual incomes
-
Bunkhouse Electronics is a recently incorporated firm that makes electronic entertainment systems. Its earnings and dividends have been growing at a rate of 38.5%, and the current dividend yield is...

Study smarter with the SolutionInn App


