Identify the best conditions for the following transformation: (a) H 2 , metal catalyst; (b) excess CH
Question:
Identify the best conditions for the following transformation:
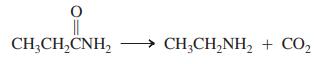
(a) H2, metal catalyst;
(b) excess CH3I, K2CO3;
(c) Br2, NaOH, H2O;
(d) LiAlH4, ether;
(e) CH2N2, ether.
Transcribed Image Text:
CH;CH,CNH, CH;CH,NH, + CO2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
The above reaction is called hoffman bromamide degradation reactionSo the bes...View the full answer

Answered By

Alex Chacko
I am Alex Chacko, a second year integrated Msc Chemistry student at Institute for intensive research in basic science (IIRBS).I have also been working as a question and answer expert with chegg for the past two years.I have been answering difficult questions for the past 2 years and hence i assure you to provide the best quality answer.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Predict the products from reaction of 5-decyne with the following reagents: (a) H2 Lindlar catalyst (b) Li in NH3 (c) 1 equiv Br2 (d) BH3 in THF, then H2O2, OH (c) H2O, H2SO4, HgSO4 (f) Excess H2,...
-
Show the products you would expect to obtain from reaction of glyceryl trioleate with the following reagents: (a) Excess Br2 in CH2Cl2, (b) H2/Pd (c) NaOH/H2C (d) O3, then Zn/CH3CO2H (e) LiAlH4, then...
-
Compound A (C7 H11Br) is treated with magnesium in ether to give B (C7H11MgBr) which reacts violently withD2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B...
-
A car has a sticker price of $69,000. The car has a 100 hp engine and can accelerate from 0 to 60 mph in 15.8 seconds. The lease rate is 4.6%. The term of the lease is three years. The buyout is...
-
You have access to the following three spot exchange rates: $0.01/yen $0.20/krone 25 yen/krone You start with dollars and want to end up with dollars. a. How would you engage in arbitrage to profit...
-
On December 25, 2017, Mr. Jones gives Charity A 1,000 shares of stock that he bought originally for $9,000. No restrictions were placed on this donation. The governing board does not want to...
-
Flintstones Corporation made credit sales of $30,000 which are subject to 6% sales tax. The corporation also made cash sales which totaled $19,610 including the 6% sales tax. (a) Prepare the entry to...
-
The projected October 31, 2011, balance sheet for Blanco Co. follows: ASSETS Cash .............................. $28,000,000 Accounts Receivable (net of Allowance for Uncollectibles of...
-
show the following transactions and how it will impact the income Statement and Balance sheet for the month of Januaary - assume all beginning balances are $0 The Sampany has a Zero Balance Account...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Employee Number Name and Address Payroll information...
-
One of the following four amines is tertiary. Which one? (a) Propanamine; (b) N-methylethanamine; (c) N,N-dimethylmethanamine; (d) N-methylpropanamine.
-
Rank the basicities of the following three nitrogen-containing compounds (most basic first): A: NH 3 B: CH 3 NH 2 C: (CH 3) 4 N + NO 3 - (a) A > B > C; (b) B > C > A; (c) C > A > B; (d) C > B > A;...
-
Provide a brief description of value-added selling. What economic forces have motivated companies to adopt value-added selling?
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
1. What gives stainless steels their good corrosion resistant properties? 2. Which stainless steel is the lowest cost and why? 3. What are some characteristics of Nickel Alloys? 4. What are the 2...
-
Problem 4. Determine the motion of a two-dimensional linear oscillator of potential energy V = kr
-
5 Informatics solutions in the "complex and catastrophic" end of the population-risk spectrum must support which type of services/functions? 1 point Intensive case management Wellness program
-
What are the characteristics of products that Otis Trains produces? What are order qualifiers and winners? Explain at least three advantages and three drawbacks of offshoring to JLPTC. What risks are...
-
What is the output produced from the following statements? System.out.println("\ta\tb\tc"); System.out.println("\\\\"); System.out.println("'"); System.out.println("\"\"\""); System.out.println("C:...
-
31. What is the income that can be received over 15 years from $500,000 earning 6% annually? 32. What is the semiannual payment required to retire $50,000 in debt over 5 years at 8% compounded...
-
Explain why the hydration of this alkene occurs 1015 times faster than the hydration ofethene: OH H20 CH,CH,OCHCH3 CH.CH,OCH=CH, H,SO,
-
The addition of HCl to alkynes proceeds through a vinyl cation intermediate. Explain which of the two possible vinyl cations that could be formed from the addition of HCl to propyne is morestable....
-
Suggest a mechanism for thisreaction: CH,Br Br2 CH2=CHCH CH,CH,OH H,O
-
Required information Required information Problem 7 - 7 ( Static ) Calculate depreciation of property and equipment and amortization of intangible assets ( LO 7 - 4 , 7 - 5 ) [ The following...
-
Process Costing - Weighted Average Method Work In Process 1 0 / 1 - 1 6 , 0 0 0 units Direct Material: 1 0 0 % complete $ 5 4 , 5 6 0 Conversion Cost: 1 0 % complete $ 3 5 , 5 6 0 Balance WIP 1 0 / 1...
-
A merchandising concern paid $1,600,000 for inventory. The market value of the inventory is $1,200,000. The net realizable value of the inventory is $1,300,000. The company found a new supplier for...

Study smarter with the SolutionInn App


