Write the expected major product of reaction of each of the carbonyl compounds (i) (iii) with
Question:
Write the expected major product of reaction of each of the carbonyl compounds (i) – (iii) with each of the reagents (a) – (h).
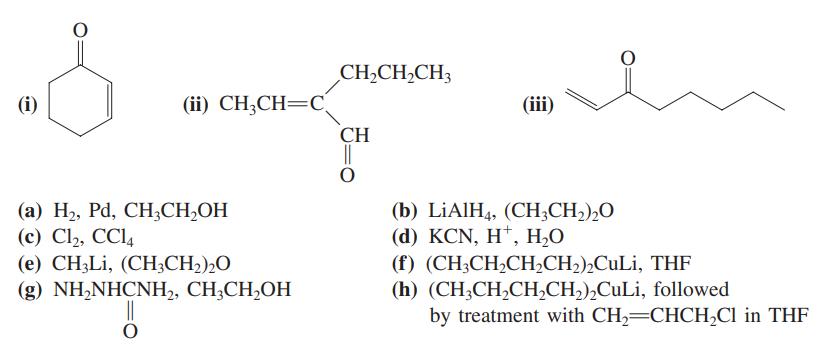
Transcribed Image Text:
CH2CH,CH3 (i) (ii) CH;CH=C. (iii) CH || (а) Н, Pа, СН,CН-ОН (c) Cl2, CCl, (e) CH;Li, (CH3CH2),O (b) LİAIH4, (CH;CH,),O (d) KCN, H*, Н.О (f) (CH;CH,CH,CH2),CuLi, THF (h) (CH3CH,CH,CH2),CuLi, followed by treatment with CH2=CHCH,Cl in THF (g) NH,NHCNH,, CH,CH,ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Write the expected major product of reaction of 1-propynyllithium, CH 3 C C - Li + , with each of the following molecules in THF. CI () C,CH,Br (b) () clohexanone CH3 (d) -H () C,CHH (f)
-
Write the expected major product(s) of each of the following attempted ether syntheses, (a) (b) (c) (d) (e) (f) DMSO CH,CH CHCI CH CH CHCH CH Cl HMPA CH CH CHO +CH,CH CHCH CH .. DMSO + CH3I (CH)...
-
Give the expected major product of reaction of 2,2-dimethyloxacyclopropane with each of the following reagents? (a) Dilute H2SO4 in CH3OH (b) Na+ -OCH3 in CH3OH (c) Dilute, aqueous HBr (d)...
-
If a particular glucose fermentation process is 87.0% efficient, how many grams of glucose would be required for the production of 51.0 g of ethyl alcohol (C 2 H 5 OH)? C 6 H 12 O 6 2C 2 H 5 OH +...
-
Explain how section 1139 found in Figure 4-2 might be used in the Welsh case.
-
Prove it for second-order PDEs in two and three independent variables. Prove it by substitution.
-
1 Do the information systems provide users with information which meets the usual criteria of high-quality information? If not, is the problem mainly technical or mainly organisational?
-
The adjusted trial balance of Pacific Scientific Corporation on December 31, 2011, the end of the company's fiscal year, contained the following income statement items ($ in millions): sales revenue,...
-
Robin Company has the following balances for the current month: Direct materials used Direct labor Sales salaries Indirect labor Production manager's salary Marketing costs Factory lease $ 9,000 $...
-
Symphony Electronics produces wireless speakers for outdoor use on patios, decks, etc. Their most popular model is the All Weather and requires four separate XL12 components per unit. The company is...
-
The distillate from sandalwood is one of the oldest and most highly valued fragrances in perfumery. The natural oil is in short supply and, until recently, synthetic substitutes have been difficult...
-
Give the expected product(s) of each of the following reactions. 1. LDA, THF 2. BICH,COCH, H 1. LDA, THF 2. CH.CH Br, HMPA (a) CH,CCH,CH,CH3 (b) 0:
-
Why shouldnt American Express have the right to cancel an account anytime? Gray could always get another card from Visa or Mastercard.
-
Use polynomial division to show that the general expression for the factors of the difference of two cubes, x 3 - y 3 = ( x - y ) ( x 2 + xy + y 2 ) , is correct
-
A beam has a lenght of L = 9 m long and carrying the uniformly distributed load of w = 3 kN/m. w kN/m A RA a) Calculate the reaction at A. RA= KN L RB b) Calculate the maximum bending moment. Mmax=...
-
3. The Balance Sheet of International Operators Ltd. as at 31.03.2021 disclose the following position: PARTICULARS SHARE CAPITAL RESERVES AND SURPLUS SECURED LOANS UNSECURED LOANS CURRENT LIABILITY...
-
A uniformly charged ring of radius a. (a) The field at P on the x axis due to an element of charge dq. (b) The perpendicular component of the field at P due to segment 1 is canceled by the...
-
At what rate would $1,000 have to be invested to grow to $4,046 in 10 years?
-
Holmes Cleaning Service began operation on January 1, Year 1. The company experienced the following events for its first year of operations: Events Affecting Year 1: 1. Provided $84,000 of cleaning...
-
Charles owns an office building and land that are used in his trade or business. The office building and land were acquired in 1978 for $800,000 and $100,000, respectively. During the current year,...
-
Azurin is a protein containing a copper ion that shuttles between the +2 and +1 oxidation states, and cytochrome c is a protein in which a haem-bound iron ion shuttles between the +3 and +2 oxidation...
-
What pressure of nitrogen gas is required to produce a collision rate of 5.00 x 1019 S-1 at 525 K on a circular surface of diameter 2.0 mm?
-
Calculate the average rate at which He atoms strike an iron atom in a surface formed by exposing a (l00) plane in metallic iron to helium gas at 100 K and a pressure of24 Pa. Crystals of iron are...
-
HBX reported the following changes in its balance sheet accounts in 2016: Accounts receivable increased by $40; accounts payable decreased by $25; accrued expenses increased by $20; net fixed assets...
-
A company is considering the purchase of a new machine for $75,660. Management predicts that the machine can produce sales of $20,000 each year for the next 10 years. Expenses are expected to include...
-
Provide an example of a detection control and a preventative control in a revenue transaction cycle of a sports club.

Study smarter with the SolutionInn App


