Consider the following four structures. a. Which of these compounds would have the same physical properties (melting
Question:
Consider the following four structures.
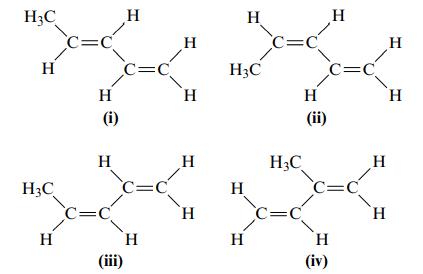
a. Which of these compounds would have the same physical properties (melting point, boiling point, density, and so on)?
b. Which of these compounds are trans isomers?
c. Which of these compounds do not exhibit cis–trans isomerism?
Transcribed Image Text:
H3C H H3C Н c=c H (i) Н c=c н c=c c=c н (iii) н H Н Н H H H3C Н H c=c H H₂C c=c c=c (ii) Н C=C H (iv) H H H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (12 reviews)
a compounds II and III have same physical pr...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the following four structures: a. Which of these compounds have the same physical properties (melting point, boiling point, density, and so on)? b. Which of these compounds are trans...
-
Which of these compounds would you expect to have the highest boiling point? Explain. [Section 24.4] CH3CH CH CH OH CHC=CH HCOCH
-
Consider the structures of cis-decalin and trans-decalin: (a) Which of these compounds would you expect to be more stable? (b) One of these two compounds is incapable of ring flipping. Identify it...
-
Stuart and Belinda, who earn good salaries, want to buy a property they can use to live in and operate a bed and breakfast in their retirement. The only funds they have available is their combined...
-
List some of the forecasting techniques that should be considered when forecasting a trending series. Give examples of situations in which these techniques would be applicable.
-
We begin with two consecutive integers, a and a + 1, such that f(a) and f(a - 1) are of opposite sign. Evaluate f at the midpoint m 1 of a and a + 1. If f(m 1 ) = 0, then m 1 is the zero of f and we...
-
how to work with data to get them ready to analyze
-
The stockholders equity for Dairy Place Drive-Ins (DP) on December 31, 2012, follows: On April 16, 2013, the market price of DP common stock was $20 per share. Assume DP distributed an 18% stock...
-
Oriole Inc. established a SARs program on January 1 , 2 0 2 3 , that entitles executives to receive cash at the date of exercise for the difference between the shares' fair value and the pre -...
-
In a survey of 130 people who used food delivery services, it was determined that 74 used Grubhub. 70 used Uber Eats. 41 used both Grubhub and Uber Eats. Of those surveyed, a) How many used only...
-
Draw the structures for two examples of unsaturated hydrocarbons. What structural feature makes a hydrocarbon unsaturated?
-
Give two examples of saturated hydrocarbons. How many other atoms are bonded to each carbon in a saturated hydrocarbon?
-
Explain two different ways of changing the speed of a movie.
-
The break even point of a company is $240 000. They sell their product at a markup of 30% and have variable expenses of 9% of sales. They currently make a profit of $10 500. They plan on reducing...
-
What kind of messages are young girls and boys receiving about whether their safety in relationships is valued are by their families and communities? Do we emphasize to our children (boys and girls)...
-
Below are the transactions for Oliver Printing, Incorporated for June, the first month of operations. June 1 Obtain a loan of $ 5 6 , 0 0 0 from the bank by signing a note. June 2 Issue common stock...
-
Assume an organization must invest $ 7 0 0 , 0 0 0 in fixed costs to produce a product that sells for $ 7 5 and requires $ 4 0 in variable costs to produce one unit. What is the organization s...
-
hotel delta marriott montreal What do you think is the value and purpose for the hotel brand choosing to make CSR an important part of their overall business strategy? What two recommendations based...
-
Explain the difference between bivariate (simple) regression and multiple regression.
-
I frequently use NY Times and CNN and am aware of Fox News but I never use it. I visit these sites, NY Times and CNN, a few times a week whenever I have to research something or see something on...
-
The following thermal rearrangement involves two pericyclic reactions in sequence. Identify them, and propose a mechanism to account for the observedresult. 275 "C CD2 - -D H2C CD2
-
Predict the product of the following pericyclic reaction. Is this [5, 5] shift a suprafacial or an antarafacialprocess? [5,5] CH eat
-
Ring-opening of the trans-Cyclobutene isomer shown takes place at much lower temperature than a similar ring-opening of the cis-Cyclobutene isomer. Explain the temperature effect, and identify the...
-
PLEASE HELP WITH PART 2 & 3 Thanks Required information Exercise 1 0 - 7 ( Algo ) Part 2 Prepare journal entries to record the first two interest payments. Journal entry worksheet Record the interest...
-
The following information was available for the year ended December 31, 2022: Net sales $ 300,000 Cost of goods sold 210,000 Average accounts receivable for the year 15,000 Accounts receivable at...
-
Oslo Company prepared the following contribution format income statement based on a sales volume of 1,000 units (the relevant range of production is 500 units to 1,500 units): Sales $ 100,000...

Study smarter with the SolutionInn App


