Consider the hypothetical reaction between A 2 and AB pictured below. What is the balanced equation? If
Question:
Consider the hypothetical reaction between A2 and AB pictured below.
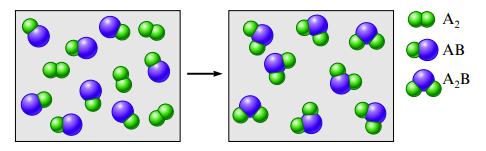
What is the balanced equation? If 2.50 moles of A2 are reacted with excess AB, what amount (moles) of product will form? If the mass of AB is 30.0 u and the mass of A2 are 40.0 u, what is the mass of the product? If 15.0 g of AB is reacted, what mass of A2 is required to react with all of the AB, and what mass of product is formed?
Transcribed Image Text:
Az AB A,B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
ANSWER Counting the number of each kind of atom on the reactants and products sides will allow us to ...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the hypothetical reaction B E + F which is assumed to occur by the mechanism Where B* represents a B molecule with enough energy to surmount the reaction energy bar70. Consider the following...
-
Consider the hypothetical reaction A + B + 2C 2D + 3E In a study of this reaction, three experiments were run at the same temperature. The rate is defined as 2d[B]/dt. Experiment 1: [A]0 = 2.0 M [B]0...
-
Consider the hypothetical reaction A + B + 2C 2D + 3E where the rate law is An experiment is carried out where [A]0 = 1.0 Ã 10-2 M, [B]0 = 3.0 M, and [C]0 = 2.0 M. The reaction is started, and...
-
There were no other non-current assets acquisitions or disposals. A dividend of 150m was paid on ordinary shares during the year. Required a) Prepare a cash flow statement for Blackheath plc for the...
-
Peter Baumann, your client, wants to sell a printing press to Chamberlain Corporation for $50,000. Pete has used the press in his business for two years and its adjusted basis is $90,000. The Coxmann...
-
Mutual gains bargaining is based on four principles. Identify these principles and explain what difference they make in the way a negotiation process is conducted.
-
Defection from a service based on payment plan. Refer to the Journal of Marketing Research (October 2019) study on defection from a service plan, Exercise 12.40 (p. 742). The researchers fit the...
-
Marc Smith's construction firm currently has three projects under way in various counties in Iowa. Each requires a specific supply of gravel. Three gravel pits are available in Iowa to provide for...
-
A $5000, 6.25% bond with interest payable annually redeemable at par in eight years is purchased to yield 7.5% compounded annually. Find the premium or discount and the purchase price and construct...
-
1) Value of agility is a key parameter when deciding TCO/ROI. True or False 2) Select the one which is not a domain factor for the interconnected systems in Cloud operations A) User domain B)...
-
What three conversion factors and in what order would you use them to convert the mass of a compound into atoms of a particular element in that compoundfor example, from 1.00 g aspirin (C 9 H 8 O 4 )...
-
True or false? The atom with the largest subscript in a formula is the atom with the largest percent by mass in the compound. If true, explain why with an example. If false, explain why with an...
-
True or False: 1. If demand is inelastic, the price and total revenue will move in opposite directions along the demand curve. 2. A straight-line demand curve will have a constant elasticity of...
-
Verify the results of Eq. (14.48) for the properties of the chiral projection operators. Data from Eq. 14.48 P = P+ P+ + P = 1 P_P+ P+P = 0 Py" = y P
-
Prove that the estimating equations in (11.13) are unbiased under MCAR, but are generally biased without the stringent MCAR assumption. (x) [y - f (xt;)] = 0, i=1 (11.13)
-
Refer to Figure 11.5: Which is the most expensive subcontract for this project? How much were the costs for the general contractor's crews for item 4? Figure 11.5 Division 1 2 3 4 5 6 7 Work Gen'l...
-
a. Using observations on the change in consumption \(D C_{t}=C_{t}-C_{t-1}\) and the change in income \(D Y_{t}=\) \(Y_{t}-Y_{t-1}\) from 1959Q3 to 2015Q4, obtained from the data file cons_inc,...
-
Water at \(20^{\circ} \mathrm{C}\) flows by gravity from a large reservoir at a high elevation to a smaller one through a 35-m-long, 5-cm-diameter cast iron piping system that includes four standard...
-
If money supply is endogenous, as Keynesians claim, what will happen to the supply of money if there is an expansionary fiscal policy? What implications does this have for the rise in interest rates?
-
MgO prevents premature evaporation of Al in a furnace by maintaining the aluminum as Al2O3. Another type of matrix modifier prevents loss of signal from the atom X that readily forms the molecular...
-
Label the regions of the phase diagram in Fig. 6.3 7. State what substances (if compounds give their formulas) exist in each region. Label each substance in each region as solid, liquid, or gas.
-
The emf of the cell Bi|Bi2S3(s) IBi2S3(aq) IBi is -0.96 V at 25e. Calculate (a) The solubility product ofBi2S3 and (b) Its solubility. at310K?
-
At 90C, the vapour pressure of I, 2-dimethylbenzene is 20 kPa and that of 1,3-dimethylbenzene is 18 kPa. What is the composition of a liquid mixture that boils at 90C when the pressure is 19 kPa?...
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


