Determine the liquidus temperature, solidus temperature, and freezing range for the following NiO-MgO ceramic compositions: (a) NiO-30
Question:
Determine the liquidus temperature, solidus temperature, and freezing range for the following NiO-MgO ceramic compositions:
(a) NiO-30 mol% MgO;
(b) NiO-45 mol% MgO;
(c) NiO-60 mol% MgO; and
(d) NiO-85 mol% MgO.
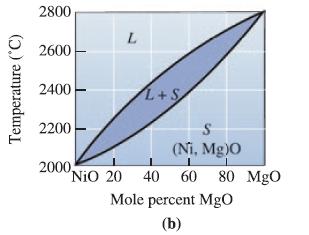
Transcribed Image Text:
Temperature (°C) 2800 2600 2400 2200 L 2000, L+S S (Ni, Mg)0 "NiO 20 40 60 80 MgO Mole percent MgO (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a The liquidus temperature 1515 K and the solidus temperature 1486 K The freezing range will occu...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following MgO-FeO ceramic compositions. (a) MgO-25 wt% FeO; (b) MgO-45 wt% FeO; (c) MgO-65 wt% FeO; (d) MgO-80 wt%...
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following Al 2 O 3 -Cr 2 O 3 ceramic compositions: (a) Al 2 O 3 -30 wt% Cr 2 O 3 ; (b) Al 2 O 3 -50 wt% Cr 2 O 3 ;...
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following MgO-FeO ceramic compositions: (a) MgO-25 wt% FeO; (b) MgO-45 wt% FeO; (c) MgO-65 wt% FeO; and (d) MgO-80...
-
How do recruitment and selection practices contribute to high performance in an organization?
-
Starting from point E in Figure 18.8, draw a figure showing how the nation could reach internal and external balance with flexible exchange rates by using an expansionary fiscal rather than an easy...
-
An envelope detector operates above threshold. The modulating signal is a sinusoid. Plot (SNR) D in decibels as a function of P T /N 0 W for the modulation index equal to 0.3, 0.5, 0.6, and 0.8.
-
On February 19, 1992, Walt Disney Co. declared a 4:1 split of its common stock, an announce ment that boosted the entertainment companys shares up $3.50 to close at a record price of $146.50....
-
Common-size and trend percents for Aziz Companys sales, cost of goods sold, and expenses follow. Determine whether net income increased, decreased, or remained unchanged in this three-yearperiod....
-
SecuriCorp operates a fleet of armored cars that make scheduled pickups and deliveries in the Los Angeles area. The company is implementing an activity-based costing system that has four activity...
-
Your company wants to invest its cash surplus of $1m. in a Treasury bill for a period of 6 months. The T-bill rate is 5.5% p.a. continuous compounding. The company's treasurer, has asked you to...
-
Determine the phases present, the compositions of each phase, and the amount of each phase in wt% for the following Al 2 O 3 - Cr 2 O 3 ceramics at 2150C: (a) Al 2 O 3 -30 wt% Cr 2 O 3 ; (b) Al 2 O 3...
-
Determine the phases present, the compositions of each phase, and the amount of each phase in wt% for the following MgOFeO ceramics at 2000C: (a) MgO-25 wt% FeO; (b) MgO-45 wt% FeO; (c) MgO-60 wt%...
-
High-power lasers are used to cut and weld materials by focusing the laser beam to a very small spot. This is like using a magnifying lens to focus the suns light to a small spot that can burn...
-
Kelly Corporation received an advanced payment of \(\$ 30,000\) in 2018 from Rufus Company for consulting services. Kelly performed half of the consulting in 2018 and the remainder in 2019. Kelly...
-
Rosa Dominguez, the owner of Elegant Dining in San Jose, California, is pondering whether to buy electronic menu technology and tablets for her five-star restaurant. Prices for a typical four course...
-
Dura Corporation makes metal frames for several world brands of portable home generators. They sell the frames to a wide variety of portable generator manufacturers such as DeWalt, DuroMax, Generac,...
-
Rocker Industries (RI) produces recreational in-line skates (see Exhibit 14.36). Demand is seasonal, peaking in the summer months, with a smaller peak demand during December. For one of their more...
-
The BOM, current inventory, and lead time (in months) for the in-line skates in Rocker Industries (A) case is shown in Exhibit 14.37. Using the chase demand strategy, you developed in Rocker...
-
Find the direction angle of v for a given vector. v = -i + 3j
-
In the figure, two loudspeakers, separated by a distance of d1 = 2.63 m, are in phase. Assume the amplitudes of the sound from the speakers are approximately the same at the position of a listener,...
-
Predict some possible compounds that could form between chlorine and selenium.
-
There are two forms of solid sulfur: rhombic and monoclinic. The stable form of sulfur at 25C is the rhombic form. Upon heating, the rhombic form converts to the monoclinic form, which is the stable...
-
Fluorine reacts with sulfur to form several different covalent compounds. Three of these compounds are SF 2 , SF 4 , and SF 6 . Draw the Lewis structures for these compounds, and predict the...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


