Determine the liquidus temperature, solidus temperature, and freezing range for the following MgO-FeO ceramic compositions: (a) MgO-25
Question:
Determine the liquidus temperature, solidus temperature, and freezing range for the following MgO-FeO ceramic compositions:
(a) MgO-25 wt% FeO;
(b) MgO-45 wt% FeO;
(c) MgO-65 wt% FeO; and
(d) MgO-80 wt% FeO.
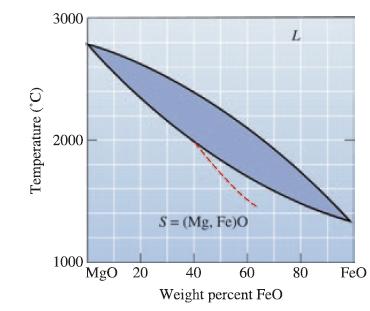
Transcribed Image Text:
Temperature (°C) 3000 2000 1000 MgO 20 S = (Mg,Fe)O 40 60 Weight percent FeO L 80 FeO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a The liquidus temperature 12283 K the solidus temperature 13023 K and the free...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following MgO-FeO ceramic compositions. (a) MgO-25 wt% FeO; (b) MgO-45 wt% FeO; (c) MgO-65 wt% FeO; (d) MgO-80 wt%...
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following NiO-MgO ceramic compositions: (a) NiO-30 mol% MgO; (b) NiO-45 mol% MgO; (c) NiO-60 mol% MgO; and (d)...
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following Al 2 O 3 -Cr 2 O 3 ceramic compositions: (a) Al 2 O 3 -30 wt% Cr 2 O 3 ; (b) Al 2 O 3 -50 wt% Cr 2 O 3 ;...
-
A 4-ft-high, 3-ft-diameter cylindrical water tank whose top is open to the atmosphere is initially filled with water. Now the discharge plug near the bottom of the tank is pulled out, and a water jet...
-
Starting with the algebraic and graphical results of Problems 5 and 6, determine algebraically and determine graphically the effect on YE of an autonomous: (a) Increase in X of 200. (b) Increase in I...
-
Common stock issues in this market with average systematic risk are most likely to have required rates of return A. Between 2 percent and 7 percent. B. Between 7 and 9 percent. C. At 9 percent or...
-
The following quotes are from The Wall Street Journal. RJR Nabisco Inc. said it plans to buy back as much as 8 percent of its outstanding stock for $52 to $58 dollars per share, or up to $1.2...
-
Martin Manufacturing decided to raise additional long-term capital by mortgaging an industrial park it owned. First National Loan Co. agreed to lend Martin $1 million and to take a note and first...
-
Racine Filter Corporation used the following data to evaluate their current operating system. The company sells items for $14.50 each and had used a budgeted selling price of $15 per unit. Actual...
-
The Tusquittee Company is a retail company that began operations on October 1, 2018, when it incorporated in the state of North Carolina. The Tusquittee Company is authorized to issue 100,000 shares...
-
Locate the following points in the Ag-Pd phase diagram and indicate the phases present and their relative amounts: (a) 50 wt% Pd at 1300C; (b) 80 wt% Pd at1425C; and (c) 90 wt% Ag at 1100C....
-
For a Ag-60 wt% Pd alloy determine the (a) Liquidus temperature; (b) Solidus temperature; and (c) Freezing range. Temperature (C) 1600 1500 1400 1300 1200 1100 1000 900 961.93 C 0 10 Ag 20 1555 C 30...
-
Assume the same probabilities as in the previous question. For a randomly selected family with three children, whats the probability of (a) Three boys? (b) Three girls? (c) Either three boys or three...
-
Customers arrive at a ferry ticket office at the rate of 14 per hour on Monday morn- ings. This can be described by a Poisson distribution. Selling the tickets and pro- viding general information...
-
Glen County manages a waste-to-energy facility that burns 2,000 tons of trash per day and generates over \($20\) million in electricity annually while costing state and local taxpayers \($24\)...
-
Carry out a full decision analysis for Classical Reproductions Ltd, using the following information: Calculation of expected profit with perfect information Prior probabilities for the various events...
-
T and B lymphocytes are normal components of the immune system, but in multiple sclerosis they become autoreactive and attack the central nervous system. What triggers the autoimmune process? One...
-
Prove (11.32) . E (Yi,k | Zi = 0, = e) = E (Yi,k | i = 1, = e) = E (Yi,k | Ti = e), k = 1,2. (11.32)
-
Find the direction angle of v for a given vector. v = -3 3 i + 3 j
-
d. The characteristic equation of a control system is given by s+2s+8s+12s+20s+16+16=0. Determine the number of the roots of the equation which lie on the imaginary axis of s-plane
-
Many lithium salts are hygroscopic (absorb water), but the corresponding salts of the other alkali metals are not. Why are lithium salts different from the others?
-
There are three known xenon fluoride covalent compounds: XeF 2 , XeF 4 , and XeF 6 . In general, the xenon fluoride compounds must be stored in an inert atmosphere, free of oxygen and water. Why is...
-
What are boranes? Why were they once considered as potential fuels for rockets?
-
i need help in B and C Integrative Case 5-72 (Algo) Cost Estimation, CVP Analysis, and Decision Making (LO 5-4.5.9) Luke Corporation produces a variety of products, each within their own division....
-
Relate PSA (Public Securities Association) speed to the average life of a MBS. Describe the PSA measure and discuss which MBS would have the greater average life, one with a PSA of 100 or one with a...
-
Which of the following statement about swaps is least accurate? A. In a plain vanilla interest rate swap, the notional principal is swapped. B. The default problem [i.e. default risk] is the most...

Study smarter with the SolutionInn App


