Locate the following points in the Ag-Pd phase diagram and indicate the phases present and their relative
Question:
Locate the following points in the Ag-Pd phase diagram and indicate the phases present and their relative amounts:
(a) 50 wt% Pd at 1300°C;
(b) 80 wt% Pd at1425°C; and
(c) 90 wt% Ag at 1100°C.
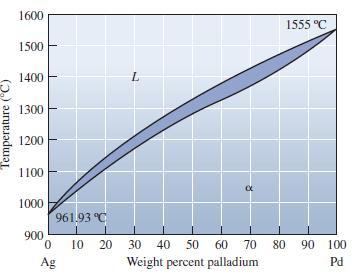
Transcribed Image Text:
Temperature (°C) 1600 1500 1400 1300 1200 1100 1000 900 961.93 °C 0 10 Ag 20 α 30 40 50 60 70 Weight percent palladium 1555 °C 80 90 100 Pd
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
a 50 w t P d at 1300 C At this point the phase present is ...View the full answer

Answered By

DHRUV RAI
As a tutor, I have a strong hands-on experience in providing individualized instruction and support to students of all ages and ability levels. I have worked with students in both one-on-one and group settings, and I am skilled in creating engaging and effective lesson plans that meet the unique needs of each student.
I am proficient in using a variety of teaching techniques and approaches, including problem-based learning, inquiry-based learning, and project-based learning. I also have experience in using technology, such as online learning platforms and educational software, to enhance the learning experience for my students.
In addition to my teaching experience, I have also completed advanced coursework in the subjects that I tutor, including mathematics, science, and language arts. This has allowed me to stay up-to-date on the latest educational trends and best practices, and to provide my students with the most current and effective teaching methods.
Overall, my hands-on experience and proficiency as a tutor have equipped me with the knowledge, skills, and expertise to help students achieve their academic goals and succeed in their studies.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Locate the following points in the Bi-Sb phase diagram and indicate the phases present and their relative amounts: (a) 60 at% Bi at 250C; (b) 30 at% Bi at 500C; and (c) 50 at% Bi at 600C. (See Figure...
-
Calculate pCu2 at each of the following points in the titration of 50.00 mL of 0.001 00 M Cu2 with 0.001 00 M EDTA at pH 11.00 in a solution with [NH3] fixed at 1.00 M: (a) 0 mL (b) 1.00 mL (c) 45.00...
-
Calculate the pH at each of the following points in the titration of 50.00 mL of 0.010 0 M NaOH with 0.100 M HCl. Volume of acid added: 0.00, 1.00, 2.00, 3.00, 4.00, 4.50, 4.90, 4.99, 5.00, 5.01,...
-
Find the dy/dx for the following 3 2x+1.2x+1 (x +1)4 1- y=-
-
Starting with the algebraic and graphical results of Problems 5 and 6, determine algebraically and determine graphically the effect on YE of an autonomous: (a) Decrease in S of 100. (b) Decrease in M...
-
An analyst wants to account for financial distress and market capitalization as well as market risk in his cost of equity estimate for a particular traded company. Which of the following models is...
-
Forbes (June 3, 1985) points out that companies wishing to protect their credit ratings and unwilling to issue more stock are raising capital by issuing hybrid securities. The article specif ically...
-
Why do you think Maury Mills got in the shape it is? What are some of the mistakes you think they may have made in managing their human resources? What would you recommend that Dana and Anne do to...
-
Feather Friends, Inc. distributes a high-quality wooden birdhouse that sells $20 per unit. Variable expenses are $8 per unit, and fixed expenses total $180,000 per year. Its operating results for...
-
Iranzo Ammunition is an all-equity firm that currently has 6,000,000 shares outstanding worth $50 per share. The company's considering converting to a capital structure that is 50.0% debt. The firm...
-
A NiO-20 mol% MgO ceramic is heated to 2200C. Determine (a) The composition of the solid and liquid phases in both mol% and wt%; (b) The amount of each phase in mol% and wt%; and (c) Assuming that...
-
Determine the liquidus temperature, solidus temperature, and freezing range for the following MgO-FeO ceramic compositions: (a) MgO-25 wt% FeO; (b) MgO-45 wt% FeO; (c) MgO-65 wt% FeO; and (d) MgO-80...
-
Bill Rubble, president of Melinco Company, reviewed a proposal for a project investment that was submitted by the vice president of production. Bill attached a note to the proposal that said, "Please...
-
Verify the results of Eq. (14.48) for the properties of the chiral projection operators. Data from Eq. 14.48 P = P+ P+ + P = 1 P_P+ P+P = 0 Py" = y P
-
Prove that the estimating equations in (11.13) are unbiased under MCAR, but are generally biased without the stringent MCAR assumption. (x) [y - f (xt;)] = 0, i=1 (11.13)
-
Refer to Figure 11.5: Which is the most expensive subcontract for this project? How much were the costs for the general contractor's crews for item 4? Figure 11.5 Division 1 2 3 4 5 6 7 Work Gen'l...
-
a. Using observations on the change in consumption \(D C_{t}=C_{t}-C_{t-1}\) and the change in income \(D Y_{t}=\) \(Y_{t}-Y_{t-1}\) from 1959Q3 to 2015Q4, obtained from the data file cons_inc,...
-
Water at \(20^{\circ} \mathrm{C}\) flows by gravity from a large reservoir at a high elevation to a smaller one through a 35-m-long, 5-cm-diameter cast iron piping system that includes four standard...
-
Find the direction angle of v for a given vector. v = -5i - 5j
-
1. Following are information about Alhadaf Co. Cost incurred Inventory Purchases Sales Adverting expense Salary Expense Depreciation Beginning Inventory Ending Inventory Amount 118,000 350.000 90,000...
-
Elemental boron is produced by reduction of boron oxide with magnesium to give boron and magnesium oxide. Write a balanced equation for this reaction.
-
Thallium and indium form +1 and +3 oxidation states when in compounds. Predict the formulas of the possible compounds between thallium and oxygen and between indium and chlorine. Name the compounds.
-
Consider element 113, Nh. What is the expected electron configuration for Nh? What oxidation states would be exhibited by Nh in its compounds?
-
Due to the relationship of financial statements, the statement of stockholders' equity links the income statement to the balance sheet. True or False?
-
Troy Engines, Limited, manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Trey is single and has no qualifying child. His adjusted gross income is $12,355. In order to claim the Earned Income Tax Credit, he must meet which of the following requirements? He cannot be the...

Study smarter with the SolutionInn App


