Consider the reaction of air to form a gaseous mixture containing N 2 , O 2 ,
Question:
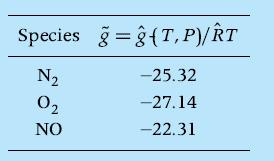
Consider the reaction of air to form a gaseous mixture containing N2, O2 , and NO and assume that the perfect gas law is applicable. Calculate the mole fractions of the species at equilibrium at T = 2000 K and P = 5 atm using the element potentials method. The nondimensional Gibbs function values of the pertinent species at these conditions are
Transcribed Image Text:
Species ĝT,P)/RT N₂ 02 NO -25.32 -27.14 -22.31
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
The values of the nondimensional Gibbs function Species T PRT N 2532 2714 2231 0 NO compare exactly ...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
A gaseous mixture containing 1.00 mol each of CO, H2O, CO2, and H2 is exposed to a zinc oxidecopper oxide catalyst at 1000oC. The reaction is and the equilibrium constant Kc is 0.58 at 1000oC. What...
-
Consider the reaction of 1 mol H2(g) at 25oC and 1 atm with 1 mol Br2(l) at the same temperature and pressure to produce gaseous HBr at these conditions. If this reaction is run in a controlled way...
-
Consider the reaction of 2 mol H2(g) at 25oC and 1 atm with 1 mol O2(g) at the same temperature and pressure to produce liquid water at these conditions. If this reaction is run in a controlled way...
-
What items should each staff member receive when beginning a veterinary job position?
-
The U.S. Office of Management and Budget (OMB) recommends that the government use different discount rates for public investments than for the sale of government assets. For public investments, the...
-
What type of questions would one expect to find on a survey of opinions about business ethics? Illustrate, with examples, their purpose, wording, and coding. Would the topics in a business ethics...
-
Suppose two assets satisfy a statistical factor model with a single factor: R 1 = E[R 1] + f + 1 , R 2 = E[R 2] f + 2, where E[f] = E[ 1] = E[2] = 0, var(f) = 1, cov(f ,1) = cov(f ,2) = 0, and...
-
Devin Advertising Companys trial balance at December 31 shows Supplies $6,700 and Supplies Expense $0. On December 31, there are $2,500 of supplies on hand. Prepare the adjusting entry at December...
-
Common-Size Financial Statement Analysis Using Excel Case scenario: You are working at a small business that has been mostly stable. You want to analyze the financial statements of the past five...
-
Your client, Leona Ledford, was personally served with a summons and complaint on October 23 in the case of Masters v Ledford Her answer is due in 30 days. You will mail the answer to the court. What...
-
One of the production processes of gallium-arsenide used in the semiconductor industry involves the equilibrium reaction of a gas mixture of As 2 , As 4 , and Ga at 800 K and 0.1 atm, thus the...
-
Consider the reaction (a) At the peak combustion temperature in a car (2500 to 2800 K), and neglecting the effect of pressure, will this reaction affect the production of NO?? (b) If the peak...
-
Use the example of Mr. Figg to explain the concepts of efficient taxes and excess burden.
-
Explain the role of EHR healthcare technology in the delivery of care
-
In the movie, Money Ball what was the change that the Oakland A's was going through under the leadership of Billy Beane? 2.: In leading the change that you described in Q1, what was the...
-
Studies of the grapevine network within organizations have shown that the rumours and gossip on the grapevine are almost always accurate, and that a prudent manager is wise to act on that...
-
What is Program Evaluation? Describe What is need assessment? Describe? What is a program logic model? Describe and analyze. What is one example? (including input, output, short term outcomes and...
-
What are the primary jobs that must be performed at Spotify? Using the job characteristics theory as a frame-work, assess these jobs in terms of their motivating potential. 2. How does the concept of...
-
Exhibit 3.6 indicates that in 1999, Germany had a current account deficit and at the same time a capital account deficit. Explain how this can happen.
-
Why is it necessary to study the diffusion of molecules in biological systems?
-
Ascorbic acid (vitamin C, page 354) reacts with I - 3 according to the equation Starch is used as an indicator in the reaction. The end point is marked by the appearance of a deep blue starch-iodine...
-
A solution of NaOH was standardized by titration of a known quantity of the primary standard, potassium hydrogen phthalate The NaOH was then used to find the concentration of an unknown solution of H...
-
Write the names and abbreviations for each of the prefixes from 10 -24 to 10 24 . Which abbreviations are capitalized?
-
Question 3 (24 marks) Wonderful Technology Company Limited sells computers and accessories. Data of the store's operations are as follow: Sales are budgeted at $400,000 for December 2019, $420,000...
-
Kratz Manufacturing Company uses an activity-based costing system. It has the following manufacturing activity areas, related cost drivers and cost allocation rates: Activity Cost Driver Cost...
-
You are a Partner with Fix-It Consultants and have been engaged in an advisory capacity with a software company, called MoveFast. The company is seeing a sharp decline in revenue, with the primary...

Study smarter with the SolutionInn App


