Question: Using data for standardized enthalpy and absolute entropy obtained from GASMIX calculate the equilibrium constant for the gaseous reaction ?298 K. Derive an expression which
Using data for standardized enthalpy and absolute entropy obtained from GASMIX calculate the equilibrium constant for the gaseous reaction ![]() ?298 K. Derive an expression which would let you obtain this, as an alternative to using Table D.1 in terms of the equilibrium constants for simpler reactions.
?298 K. Derive an expression which would let you obtain this, as an alternative to using Table D.1 in terms of the equilibrium constants for simpler reactions.
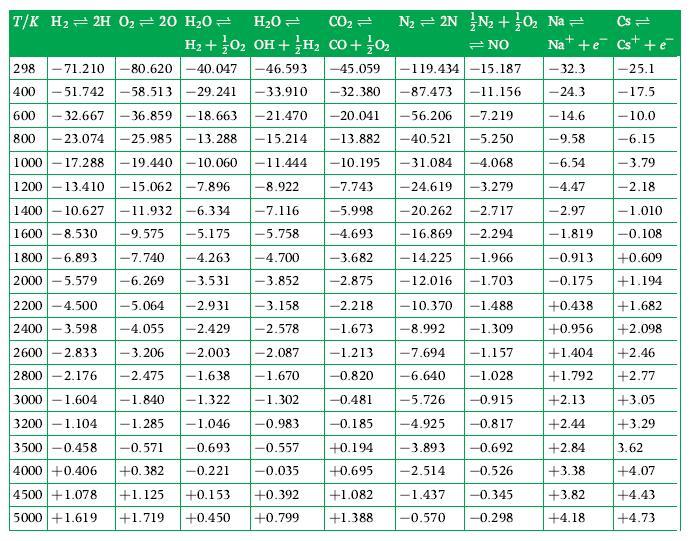
CO,+H, CO+H,O
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Given the chemical equation COH COHO and the equilibrium constant relation ... View full answer

Get step-by-step solutions from verified subject matter experts


