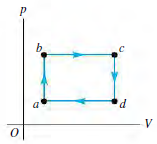
You are conducting experiments to study prototype heat engines. In one test, 4.00 mol of argon gas
Question:
(a) Calculate the efficiency e of the cycle.
(b) Disappointed by the cycle€™s low efficiency, you consider doubling the number of moles of gas while keeping the pressure and volume the same. What would e be then?
(c) You remember that the efficiency of a Carnot cycle increases if the temperature of the hot reservoir is increased. So, you return to using 4.00 mol of gas but double the volume in states c and d while keeping the pressures the same. The resulting temperatures in these states are Tc = 760.0 K and Td = 633.4 K. Ta and Tb remain the same as in part (a). Calculate e for this cycle with the new Tc and Td values.
(d) Encouraged by the increase in efficiency, you raise Tc and Td still further. But e doesn€™t increase very much; it seems to be approaching a limiting value. If Ta = 250.0 K and Tb = 300.0 K and you keep volumes Va and Vb the same as in part (a), then Tc/Td = Tb/Ta and Tc = 1.20Td. Derive an expression for e as a function of Td for this cycle. What value does e approach as Td becomes very large?
Figure P20.57
Step by Step Answer:

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman





