Using an annual interest rate (r=4 %), recompute Table 2.1 for (F V(T)) for a horizon date
Question:
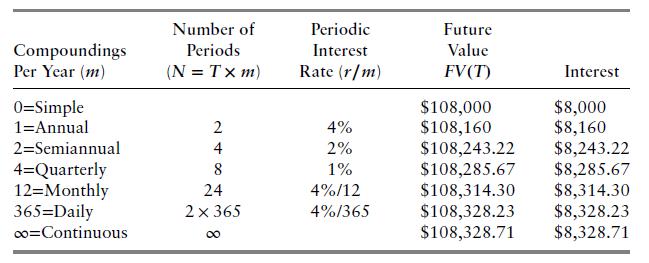
Using an annual interest rate \(r=4 \%\), recompute Table 2.1 for \(F V(T)\) for a horizon date of 6 months, \(T=0.5\). Repeat for a horizon date of \(1 \mathrm{y}\) and 6 months, \(T=1.5\).
Table 2.1
Transcribed Image Text:
Compoundings Per Year (m) 0=Simple 1=Annual 2=Semiannual 4=Quarterly 12=Monthly 365 Daily co=Continuous Number of Periods (N = Tx m) 2 4 8 24 2 365 Periodic Interest Rate (r/m) 4% 2% 1% 4%/12 4%/365 Future Value FV (T) $108,000 $108,160 Interest $8,000 $8,160 $108,243.22 $8,243.22 $108,285.67 $8,285.67 $108,314.30 $8,314.30 $108,328.23 $8,328.23 $108,328.71 $8,328.71
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Mathematical Techniques In Finance An Introduction Wiley Finance
ISBN: 9781119838401
1st Edition
Authors: Amir Sadr
Question Posted:
Students also viewed these Business questions
-
In the technique of MBO: i) Overall objectives are set at the top of the organisational hierarchy ii) Individual objectives coincide with objectives of the organisation iii) Performance should be...
-
What is the spring cloud API Gateway and how do you set it up to make a call to the product service via eureka service discovery server? Give me the complete code in detailed steps.
-
Design a controller to stabilize the system G(S) = with feedback H(S) +371
-
What problems may be encountered in making a comparative study of remuneration reports?
-
Darcie Warner, an analyst at the U.S. Census Bureau, is researching why the percentage of households without access to the internet varies by state. This measure (NOINT) will be the dependent...
-
Assume that Alshare Company uses a periodic inventory system and has these account balances: Purchases $450,000; Purchase Returns and Allowances $11,000; Purchase Discounts $8,000; and Freight-in...
-
A study of how fast a virus would spread in a metropolitan area
-
A table of data for a library is shown in the table for Problem. Normalize these data into the third normal form, preparing it for use in a relational database environment. The librarys computer is...
-
HW Problem 2.3-Prepare financial statements for Yo Co. for the year ending 12/31/X1
-
How long does it to take to double your money at a given rate \(r\) ? Begin with \[F V=P V e^{r t}=2 P V\] and approximate \(\ln (2) \approx 0.72\) to come up with the Rule of 72 . (a) At \(3 \%\),...
-
Use the Binomial Formula \[(a+b)^{n}=\sum_{k=0}^{n}\left(\begin{array}{l}n \\k\end{array}ight) a^{k} b^{n-k}\] to show (a) \[e=\lim _{n ightarrow...
-
On December 2, 2006, Pfizer Inc. announced that it was immediately discontinuing clinical trials of its Torcetrapib drug. Subsequent clinical results showed that patients taking the drug suffered a...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Multiple Choice Questions 1. Deductible expenses for moving do not include: a. The cost of transporting household goods. b. Hotel costs while moving to the new location. c. Meals incurred during the...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Why do you think Google was adamant about not wanting to supply information requested by the government concerning the Child...
-
Consider three countries: Jovenia (average age: 25), Mittelaltistan (average age: 45), and Decrepetia (average age: 75). Based on the lifecycle theory, which of these countries will probably have: a....
-
Sometimes, in supply and demand models, its not clear who supplies and who demands. For instance, in the labor market, it is individual workers (not firms) who supply labor. In the loanable funds...
-
Lets take a look at financial intermediation and the financial crisis of 20072009. Using the FRED economic database ( https://fred.stlouisfed.org/ ), search for all sectors total loans; you should...
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


