Suggest methods for preparing these compounds from alkylhalides: CN CH3 c) CH;C=CCH Ph b) HC=CCH,CH,CHCH3 a) CH3
Question:
Suggest methods for preparing these compounds from alkylhalides:
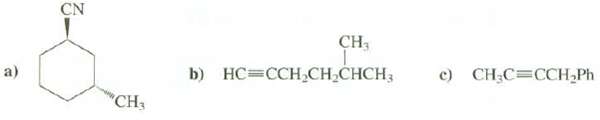
Transcribed Image Text:
CN CH3 c) CH;C=CCH Ph b) HC=CCH,CH,CHCH3 a) "CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
a J CH3 NaCN D...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Suggest appropriate methods for preparing each of the following compounds from the starting material of your choice. (a) CH3CH2CH2CH2CH2MgI (b) CH3CH2 CPCMgI (c) CH3CH2CH2CH2CH2Li (d)...
-
Suggest carbonyl compounds and reducing agents that might be used to form the following alcohols. (a) octan-1-ol (b) 1-cyclohexylpropan-1-ol (c) 1-phenylbutan-1-ol (d) (e) (f) OCH
-
Suggest a preparation for the following compounds from the appropriate alcohol. Isobutyl toslate
-
1. What are some other ways in which HEALTHeLINK could be used to support public health activities?
-
Speedy Copy Centre operates a chain of photocopy centres near several major universities. The firm's accountant is accumulating data to be used in preparing its annual budget for the coming year. The...
-
Quatro Co. issues bonds dated January 1, 2017, with a par value of $400,000. The bonds' annual contract rate is 13%, and interest is paid semiannually on June 30 and December 31. The bonds mature in...
-
_____________ is an uncertainty that can have a negative or positive effect on meeting project objectives. a. Risk utility b. Risk tolerance c. Risk management d. Risk LO.1
-
Respond to each of the following comments that you heard related to the audit of Swan Company, a public entity. a. We dont need to consider the risk of material misstatement in our work because we...
-
On pages 375-376, the text discusses the security market line, also known as the CAPM. The Capital Asset Pricing Model (CAPM) states that expected return of a stock portfolio is based on the...
-
Examine the Al literature to discover whether the following tasks can currently be solved by computers: a. Playing a decent game of table tennis (ping-pong). b. Driving in the center of Cairo. c....
-
Show the products of thesereactions: CH,CI 1) NANH2, NH3 (/), NACN b) CH,C3C- a) 2) CH,CH,CH,Br DMSO Br NaCN c) DMSO 1) NANH, 2) CH,I I) NANH2, NH, (1) d) HC=CH 2) CH,CH,Br DMF Br + I NACN e) Cl- Br...
-
Show the products of thesereactions: CH O PhS Na b) Ph,P + CH;CH;CH,Br benzene a) ELOH + CH;I c) CH;CH,CH,CH,S d) NASCH,CH2SNA + BRCH,CH,Br CH
-
If an interest rate of 6.9% compounded semi- annually is charged on a car loan, what effective rate of interest should be disclosed to the borrower?
-
use z scores to compare the given values. Pulse Rates Based on Data Set 1 "Body Data" in Appendix B, males have pulse rates with a mean of 69.6 and a standard deviation of 11.3; females have pulse...
-
(All answers were generated using 1,000 trials and native Excel functionality.) At a local university, the Student Commission on Programming and Entertainment (SCOPE) is preparing to host its first...
-
50 45 40 35 30 25 20 15 10 5 Graph 1 Percent of observers that reported seeing individual consumers exhibit the behavior under consideration Calmness Courteousness Happiness Anxiety Excitement...
-
ProForm acquired 60 percent of ClipRite on June 30, 2020, for $1,140,000 in cash. Based on ClipRite's acquisition-date fair value, an unrecorded intangible of $400,000 was recognized and is being...
-
Find the regression equation, letting the first variable be the predictor (x) variable. Using the listed lemon/crash data, where lemon imports are in metric tons and the fatality rates are per...
-
In conjunction with which measure of central tendency would you expect to report the standard deviation: the mean; the median; or the mode?
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
Solve each inequality and graph its solution. 7(x+2) < -5x (4-3x) + + + --4 -3 -2 -1 0 1 2 3 4 5 6 +
-
Assign R or S configuration to the following molecule, write the product you would expect from SN2 reaction with NaCN, and assign R or S configuration to the product (yellow-green =Cl):
-
Draw the structure and assign Z or F stereochemistry to the product you expect from E2 reaction of the following molecule with NaOH (yellow-green =Cl):
-
Which compound in each of the following pairs will react faster in an SN2 reaction with OH? (a) CH3Br or CH3I (b) CH3CH2I in ethanol or in dimethyl sulfoxide (c) (CH3) 3CC1 or CH3C1 (d) H2C = CHBr or...
-
Harper, Inc, acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's net assets on that date was $610.000, although...
-
Need a help for this! The following information is obtained from the records of Kaiser Company: On January 1, 2017 the following machines were acquired for cash: Production machines costs $ 6,000...
-
Book 51,500 Hint rint rences Raw Materials Inventory Debit Credit Beginning 10,100 Purchases 45,500 Available for use 55,600 DM used Ending 4,100 Work in Process Inventory Debit Credit Beginning...

Study smarter with the SolutionInn App


