Question: Tai and Chen [AIChE J., 41, 68-77 (1995)] studied the precipitation of calcium carbonate by mixing aqueous solutions of sodium carbonate and calcium chloride in
Tai and Chen [AIChE J., 41, 68-77 (1995)] studied the precipitation of calcium carbonate by mixing aqueous solutions of sodium carbonate and calcium chloride in an MSMPR crystallizer with pH control, such that the form of CaCO3 was calcite rather than aragonite or vaterite. In Run S-2, which was conducted at 30oC, a pH of 8.65, and 800 rpm, with a residence time of 100 min, the crystal population density data were as shown in Figure. Because the data do not plot as a straight line, they do not fit (17-38).
(a) Develop an empirical equation that will fit the data and determine, by regression, the constants.
(b) Can nucleation rate and growth rate be determined from the data? If so,how?
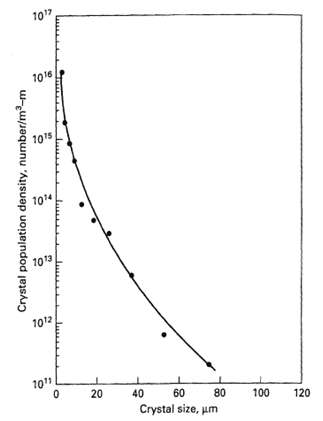
10" 1016 10'5 104 1013 1012 1011 20 40 60 80 Crystal size, um 100 120 Crystal population density, number/m-m
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
a From Fig 1737 values of n in nucleimm 3 for crystal sizes L are read an... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (592).docx
120 KBs Word File


