The compound whose 1H NMR spectrums is shown has the molecular formula C3H6Br2. Propose astructure. TMS O
Question:
The compound whose 1H NMR spectrums is shown has the molecular formula C3H6Br2. Propose astructure.
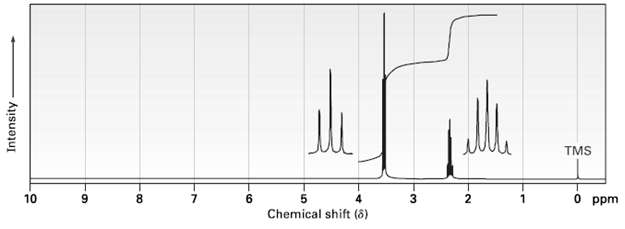
Transcribed Image Text:
TMS O ppm 10 8. 6. 1. Chemical shift (8) Intensity 6.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (23 reviews)
The unknown compound has no ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The compound whose 1 H NMR spectrum is shown has the molecular formula C 4 H 7 O 2 C1 and has an infrared absorption peak at 1740 cm ?1 . Propose a structure. TMS 10 O ppm Chemical shift (8) Intensity
-
Compound M has the molecular formula C9H12. The 1H NMR spectrum of M is given in Fig. 14.29 and the IR spectrum in Fig. 14.30. Propose a structure for M.
-
The 1H NMR spectra of three isomers with molecular formula C4H9Br are shown here. Which isomer produces which spectrum? a. b. c. (ppm) -frequency o (ppm) frequency 5 2 (ppm) frequency
-
Tom Lamont, age 30, and Lin Lamont, age 31, have been married for six years. They got married right after Tom graduated from college. They have come to you for help in planning their financial...
-
Suppose that Arnold Schwarzenegger (GAS) pays Besanko, Dranove, and Shanley (BDS) an advance of $5 million to write the script to Incomplete Contract, a movie version of their immensely popular text...
-
Refer to Exercise 3. Resistors are randomly selected from the box, one by one, until a 100 resistor is selected. a. What is the probability that the first two resistors are both 50 ? b. What is the...
-
Describe the different types of contractors.
-
Saunders Corp. has a book net worth of $13,205. Longterm debt is $8,200. Net working capital, other than cash, is $4,205. Fixed assets are $17,380. How much cash does the company have? If current...
-
Recording Bond Retirement On March 1, 2020, Sandollar Inc. issued $48,000 of bonds at 105, paying 8% cash interest semiannually on June 30 and December 31. The bonds are scheduled to mature December...
-
For the circuit in Fig. 9.73, calculate ZT and Vab. 20 ab 40 5
-
How could you use 1H NMR, 13C NMR, and IR spectroscopy to help you distinguish between the followingstructures? "CH 3-Methyl-2-cyclohexenone 3-Cyclopentenyl methyl ketone
-
Propose structures for compounds that fit the following 1H NMR data: (a) C5H10O 0.95 (6 H, doublet, J = 7 Hz) 2.10 (3 H, singlet) 2.43 (1 H, multiplet) (b) C3H5Br 2.32 (3 H, singlet) 5.35 (1 H,...
-
What is meant by the terms brain drain and reverse brain drain? q-1
-
Dr. Stanley and his staff are attempting to utilize effective ways to both increase the revenue for the practice and allow patients to schedule visits without a lengthy delay. Which of the following...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
Make a list of concepts that college students might want to learn more about. For example, a satisfaction assessment of your professors teaching approach, or an evaluation of the features and...
-
Determine whether the lines are parallel, perpendicular, or neither. 2x + 3y = -12, 2y - 3x = 8
-
Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pressure of 1.0 atm and a mole fraction for nitrogen of 0.78.
-
Show how you would use the Williamson ether synthesis to prepare the following ethers. You may use any alcohols or phenols as your organic starting materials. (a) cyclohexyl propyl ether (b)...
-
Rank each group of compounds in order of increasing heat of hydrogenation. (a) hexa-1, 2-diene; hexa-1, 3, 5-triene; hexa-1, 3-diene; hexa-1, 4-diene; hexa-1, 5-diene; hexa-2, 4-diene. (b)
-
When N-bromosuccinimide is added to hex-1-ene in CCl4 and a sunlamp is shone on the mixture, three products result. (a) Give the structures of these three products. (b) Propose a mechanism that...
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


