The key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium
Question:
The key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete thesynthesis?
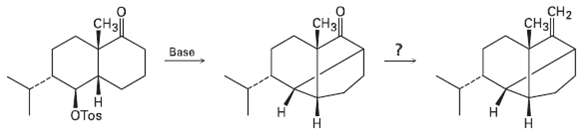
Transcribed Image Text:
CH2 CHз] CHз CH3| Base Н OTos н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
The key step is an intramolecular alkylation reaction of ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The industrial synthesis of methyl tert-butyl ether involves treatment of 2-methylpropene with methanol (CH3OH) in the presence of an acid catalyst, as shown in the following equation. CH3 H3C H3C...
-
How would you shield an electronic circuit or laboratory from stray electric fields? Why does this work?
-
The initial steps of a laboratory synthesis of several prostaglandins reported by E. J. Corey (Section 7.15B) and co-workers in 1968 are outlined here. Supply each of the missing reagents: (a) (b)...
-
Matching Financial Statement Items to Financial Statement Categories According to its annual report, P&Gs more than 250 brands include Pampers, Tide, Ariel, Always, Whisper, Pantene, Bounty,...
-
Why should you state facts in a claim message logically, objectively, and unemotionally?
-
Fig. 6.61 shows an alternate scheme for biasing the NE5234 output stage . Ignoring all base currents, find |I C74 | I C75 . Use Fig. 6.42 for the relative emitter areas of all the transistors except...
-
2. Use limit theorems to show that the following functions are continuous on [0,1]. (a) 2 f(x) = xeX + 5. (b) f(x) = 1 - x. l+x (c) f(x} ~ {:XSin~ x=t'=O x = O. (d) f(x) = Jf=X.
-
Condensed balance sheet and income statement data for Jergan Corporation are presented here. Additional information: 1. The market price of Jergans common stock was $7.00, $7.50, and $8.50 for 2017,...
-
The source document that captures how much time a worker has spent on various jobs during the period is a: Multiple Choice O job cost sheet. cost driver sheet. labor time ticket. materials...
-
Forrest runs Y Not Flowers, Inc. (YNF), a wholesale flower distributor with stores in several major metropolitan areas of the U.S. He is considering expanding his business, but he thinks his current...
-
The final step in an attempted synthesis of laurene, a hydrocarbon isolated from the marine alga Laurencia gianduliferu, involved the Wittig reaction shown. The product obtained, however, was not...
-
Amino acids can he prepared by reaction of alkyl halides with diethyl acetamidomalonate, followed by heating the initial alkylation product with aqueous lid. Show how you would prepare alanine,...
-
According to' the U.S. Census Bureau, in 2011 about 10% of persons between 25 and 39 years old live alone. For a random sample of size n, use the binomial table to find the probability of (a) 1 or...
-
Below are listed some additional common performance measures not listed in Exhibit 2.1. Which type of employee (senior managers, middle managers, or frontline operations managers) would typically use...
-
If you have a steam distillation system with immiscible organic and water phases plus a vapor phase, two volatile organic compounds plus a nonvolatile organic compound, at equilibrium how many...
-
An auditor is using difference estimation for the confirmation of accounts receivable in the audit of Lafferty Hardware Supply. A random sample of 100 positive confirmations has been sent to...
-
Canterbury Convenience Stores (CCS) is a newly formed organization in Christchurch, New Zealand. It comprises 10 moderately sized convenience stores that previously operated independently of each...
-
Orchard Distributions Pte. Ltd. is a large, Singaporean-based distributor of clothing products to other companies throughout Southeast Asia. Orders are received from customers either by telephone,...
-
I let people know in various ways that I like to be left alone to do my job efficiently. 1 2 3 4 5 LO.1
-
The population of Detroit, Michigan, decreased from 1,027,974 in 1990 to 688,701 in 2013 (Source: U.S. Census Bureau). Find the average rate of change in the population of Detroit, Michigan, over the...
-
Evidence for the additional stabilization of certain electron configurations comes from the experimental lattice energies of the metal fluorides, MF 2 . The first figure below plots lattice energy...
-
Give the structure of the compound C7H5O2Cl that has an IR absorption at 1685 cm-1 as well as a strong, broad O-H absorption, and the following proton NMR spectrum: 7.56 (2H, leaning d, J = 10 Hz); ...
-
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.51. OC,H, dil. HCI (catal ,- OC2Hs an orthoester 0 CIH O C CH dil HOI (catalyst) CH OH (e) H,C ,--, carbon monoxide CH Ph Ph...
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


