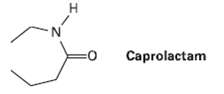
The step-growth polymer nylon 6 is prepared from caprolactam. The reaction involves initial reaction of caprolactam with
Question:
The step-growth polymer nylon 6 is prepared from caprolactam. The reaction involves initial reaction of caprolactam with water to give an intermediate open?chain amino acid, followed by healing to form the polymer. Propose mechanisms for both steps, and show the structure of nylon 6.
Transcribed Image Text:
H. Caprolactam
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Step 1 Water opens the caprolactam ring to form the amino acid ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Sodium reacts with water to give sodium hydroxide and gaseous hydrogen.
-
The amino acid cysteine has the structure shown: (a) A second sulfur-containing amino acid called cystine (C6H12N2O4S2) is formed when cysteine undergoes biological oxidation. Suggest a reasonable...
-
The amino acid cysteine has the structure shown:
-
Garcia Corporation has paid 60 consecutive quarterly cash dividends (15 years). The last 6 months, however, have been a cash drain on the company, as profit margins have been greatly narrowed by...
-
Select a business topic that interests you. Prepare a bibliography of at least five current magazine or newspaper articles, three books, and five online references that contain relevant information...
-
1. The average distance in kilometers from a school in Chicago to the closest fast food restaurant is 0.60 with a standard deviation of 0.45. Express each of the following distances as a z score: a....
-
11. What are the differences, if any, between the legal and tax classifications of business entities?
-
Quattro, Inc. has the following mutually exclusive projects available. The company has historically used a 4-year cutoff for projects. The required return is 11 percent. a. Compute the payback for...
-
ForestCo Shares have a beta of 0.34. If you believe that the appropriate risk free rate is 1.5% and the market risk premium is 8%, then what is the expected return on ForestCo shares? Enter your...
-
Your monthly phone bill has just arrived, and it's unexpectedly large. You decide to verify the amount by recalculating the bill based on your phone call logs and the phone company's charges. The...
-
The following reaction, called the benzilic acid rearrangement, takes place by typical carbonyl-group reactions. Propose a mechanism (Ph =phenyl). 1. NaOH, H,0 2. H30+ Ph C-C Ph Ph Ph Benzil...
-
Qiana, a polyamide fiber with a silky texture, has the following structure. What is the monomer units used in the synthesis ofQiana? flomdoCor CH2- Qiana CICH2)6C-NH- NH-
-
Describe the characteristics of common stock and analyze transactions affecting common stock. p. 555 LO1
-
Hey i need the following pages done ASAP.Please and thank you D2L Lesson 1: Electrostatics - Physics X L1Electrostatics X Course Hero +...
-
Bed & Bath, a retailing company, has two departmentsHardware and Linens. The companys most recent monthly contribution format income statement follows: Department Total Hardware Linens Sales $...
-
Use the formula g'(a) = lim. [(g(x)-g(a) )/ (x-a) ] x-->a to find g'(x) for g(x) = srqrt(x+7). Find the equation fo the tangrnt line to g(x) at x =-5
-
As shown in the figure below, an incompressible liquid flows steadily and vertically upward in a circular cross-sectional pipe. At section (1), the velocity profile over the cross-sectional area is...
-
writE a paper on teamwork 1. Share an experience with working on teamwork during your academic journey or on the job. 2. Share both positive and negative experiences. 3. What are your suggestions...
-
How are organizational resources linked to the competitive advantages and corporate performance of an organization? AppendixLO1
-
Transform the while loop from the previous exercise into an equivalent for loop (make sure it produces the same output).
-
Draw an energy diagram for the molecular orbitals of period 2 diatomic molecules. Show the difference in ordering for B 2 , C 2 , and N 2 compared to O 2 , F 2 , and Ne 2 .
-
Explain why borazole (sometimes called inorganic benzene) is a very stable compound. N:N borazole
-
Although aldehydes and ketones are weak acids, their a-hydrogens are rnore than 30 pKa units more acidic than the hydrogen's of alkanes. Using polar effects and resonance effects in your argument,...
-
Although aldehydes and ketones are weak acids, their a-hydrogens are rnore than 30 pKa units more acidic than the hydrogen's of alkanes. Using polar effects and resonance effects in your argument,...
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


