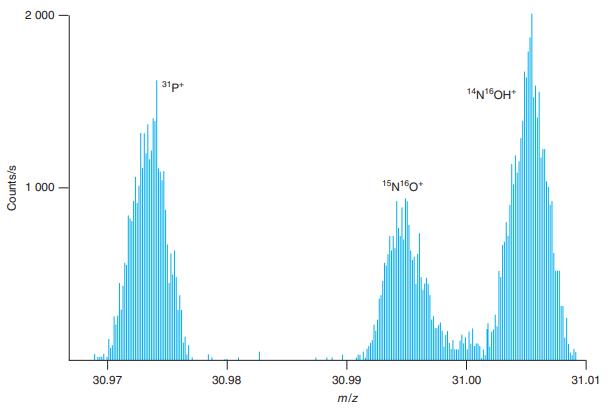
The two peaks near m/z 31.00 in Figure 21-9 differ in mass by 0.010 Da. Estimate the
Question:
The two peaks near m/z 31.00 in Figure 21-9 differ in mass by 0.010 Da. Estimate the resolving power of the spectrometer from the expression m/∆m without making any measurements in the figure.
Figure 21-9
Transcribed Image Text:
2 000 31p 14N1OH* 1 000 15N10* 30.97 30.98 30.99 31.00 31.01 m/z Counts/s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
The overlap at the base of ...View the full answer

Answered By

Sufiyan Ahmed Tariq
I am a Chartered Accountant and an Associate Public & Finance Accountant. I also hold a bachelors of Commerce degree. I have over 8 years of experience in accounting, finance and auditing. Through out my career, I have worked with many leading multinational organisation.
I have helped a number of students in studies by teaching them key concepts of subjects like accounting, finance, corporate law and auditing. I help students understanding the complex situation by providing them daily life examples.
I can help you in the following subject / areas:
a) Accounting;
b) Finance;
c) Commerce;
d) Auditing; and
e) Corporate Law.
4.90+
7+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Which of these compounds has two strong peaks near 1520 and 1350 cm1 in its IR spectrum?
-
FIGURE CP33.73 shows two nearly overlapped intensity peaks of the sort you might produce with a diffraction grating (see Figure 33.9b). As a practical matter, two peaks can just barely be resolved if...
-
In Figure horizontal scaffold 2, with uniform mass m2 = 30.0 kg and length L2 = 2.00 m, hangs from horizontal scaffold 1, with uniform mass m1 = 50.0 kg. A 20.0 kg box of nails lies on scaffold Z,...
-
Consider three (ideally other) countries for which evidence features here. What are the key influences on cross-national comparative variation in the place and role of the HRM function?
-
Weber Limited is trying to determine the value of its ending inventory at February 28, 2014, the companys year-end. The accountant counted everything that was in the warehouse as of February 28,...
-
Identify three start-ups, other than those discussed in the chapter or listed in Table 2.1, which were started to satisfy a changing environmental trend. Briefly describe the start-up and the...
-
Is the time frame for achieving the projects MOV always the same as the scheduled delivery of the projects product, service, or system? AppendixLO1
-
Suppose a customer rents a vehicle for three months from Commodores Rental on November 1, paying $6,000 ($2,000/month). (1) Record the rental for Commodores on November 1. (2) Record the adjusting...
-
1. Cool Pool has these costs associated with production of 19,000 units of accessory products: direct materials, $65; direct labor, $125; variable manufacturing overhead, $55; total fixed...
-
Over a long period of time would you expect the risk-adjusted performance of conglomerate firms to be significantly different from the risk-adjusted performance of a broad market index? Explain.
-
Measure the width at half-height of the tallest peak in the spectrum below and calculate the resolving power of the spectrometer from the expression m/m 1/2 . Would you expect to be able to...
-
The highest resolution mass spectra are obtained by Fourier transform ion cyclotron resonance mass spectrometry. Molecular ions of two peptides (chains of seven amino acids) differing in mass by...
-
Explain what will happen if the steps depicted in Figure 4.4 are reversed.
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
Vitamin A is converted by the body to retinal, a compound that is critical to human sight. The skeleton structure of vitamin A follows. Write the Lewis structure. How many sp 2 and sp 3 hybridized...
-
What types of questions can be answered by analyzing financial statements?
-
A mixture containing only aluminum tetrafl uoroborate, Al(BF 4 ) 3 (FM 287.39), and magnesium nitrate, Mg(NO 3 ) 2 (FM 148.31), weighed 0.282 8 g. It was dissolved in 1 wt% HF(aq) and treated with...
-
Explain what is done in thermogravimetric analysis.
-
Why is it less desirable to wash AgCl precipitate with aqueous NaNO 3 than with HNO 3 solution?
-
Date Account and Explanation Debit Credit Jun 1 Cash 105,000 Jun 1 Capital 105,000 (capital contribution) Jun 1 Computer Equipment 56,000 Jun 1 Cash 56,000 Jun 1 Cash 198,000 Jun 1 Bank Loan Payable...
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...

Study smarter with the SolutionInn App


