Upon consideration of the SiO2???Al2O3 phase diagram, Figure, for each pair of the following list of compositions,
Question:
Upon consideration of the SiO2???Al2O3 phase diagram, Figure, for each pair of the following list of compositions, which would you judge to be the more desirable refractory? Justify your choices.
(a) 20 wt% Al2O3-80 wt% SiO2 and 25 wt% Al2O3-75 wt% SiO2
(b) 70 wt% Al2O3-30 wt% SiO2 and 80 wt% Al2O3-20 wt%SiO2
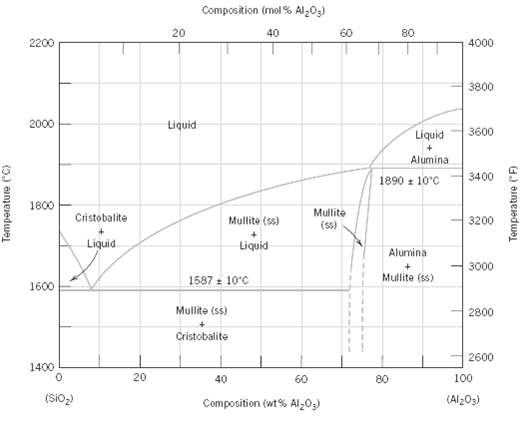
Transcribed Image Text:
Composition (mol % Al203) 80 20 40 60 2200 4000 3800 2000 Liquid Liquid 3600 Alumina 3400 1890 + 10°C 1800 Mullite (s) Cristobalite 3200 Mullite (ss) Liquid Liquid Alumina 3000 Mullite (ss) 1587 : 10°C 1600 Mulite (ss) 2800 Cristobalite 2600 1400 20 100 40 60 80 (SIO,) (Al203) Composition (wt % Al;03) Temperature ("C) Temperature ("F)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
a The 25 wt Al 2 O 3 75 wt SiO 2 will be more desirable because ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
For each pair of the following molecules, indicate whether its members are identical, structural isomers, conformers, or stereoisomers. How would you describe the relation between conformations when...
-
Upon consideration of the SiO2-Al2O3 phase diagram, Figure 12.27, for each pair of the following list of compositions, which would you judge to be the more desirable refractory? Justify your choices....
-
For each pair of the following compounds identify which compound would react more rapidly in an E1 reaction. a. b. CI .CI CI CI
-
Marsden Corp has developed a new strategic plan after rushing defective products to market hurt the company's sales and image. The strategic plan's initiatives are to focus on quality and develop...
-
Briefly describe the Religion of the Iceland. Briefly describe the Government of the Iceland. Briefly describe the Technology of the Iceland. How do these cultural elements described in this peer...
-
Maquoketa Services was formed on May 1, 2022. The following transactions took place during the first month. Transactions on May 1: 1. Jay Bradford invested $40,000 cash in the company, as its sole...
-
Name three reasons why managers should analyze their firms financial statements. LO.1
-
Low Carb Diet Supplement Inc. has two divisions. Division A has a profit of $156,000 on sales of $2,010,000. Division B is only able to make $28,800 on sales of $329,000. Based on the profit margins...
-
What periodic payment does Imran receive from a $100 000, 3-year, monthly payment GIC earning a nominal rate of 2.25% payable monthly?
-
The Golden Nursery School Company provides baby-sitting and child-care programs. On January 31, 2011, the company had the following trial balance; During the month of February, the company completed...
-
Find the maximum temperature to which the following two magnesia???alumina refractory materials may be heated before a liquid phase will appear. (a) A spinel-bonded alumina material of composition 95...
-
Compute the mass fractions of liquid in the following refractory materials at 1600C (2910F): (a) 6 wt% Al2O3-94 wt% SiO2 (b) 10 wt% Al2O3-90 wt% SiO2 (c) 30 wt% Al2O3-70 wt% SiO2 (d) 80 wt% Al2O3-20...
-
The case study maps out recommendations for organizations to support minoritized populations. On an individual level, what can athletic administrators, coaches, faculty, or other stakeholders in the...
-
Below are incomplete financial statements for Hurricane, Incorporated Required: Calculate the missing amounts. Complete this question by entering your answers in the tabs below. Income Statement Stmt...
-
TBTF Incorporated purchased equipment on May 1, 2021. The company depreciates its equipment using the double-declining balance method. Other information pertaining to the equipment purchased by TBTF...
-
Coco Ltd. manufactures milk and dark chocolate blocks. Below is the information relating to each type of chocolate. Milk Chocolate Selling price per unit $6 Variable cost per unit $3 Sales mix 4 Dark...
-
Data related to 2018 operations for Constaga Products, a manufacturer of sewing machines: Sales volume 5,000 units Sales price $300.00 per unit Variable production costs Direct materials 75.00 per...
-
6. (20 points) Sections 3.1-3.5, 3.7 Differentiate the following functions, state the regions where the functions are analytic. a. cos(e*) b. 1 ez +1 c. Log (z+1) (Hint: To find where it is analytic,...
-
DE26-13 Refer to Etron Contract Manufacturing in Daily Exercise 26-12. Suppose that Making the filters will require Etron to hire a new part-time supervisor at a cost of $8.000. If Etron buys the...
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
Sketch a graph of the potential energy of two atoms as a function of the distance between them. On your graph, indicate how bond energy and bond distance are defined.
-
What is the difference between a coherent precipitate and a distinct second-phase particle?
-
What is overaging?
-
What types of heating and cooling conditions are imposed in an I-T or T-T-T diagram? Are they realistic for the processing of commercial items?
-
Oct. 31: Paid salaries, $45,000 ( 75% selling, 25% administrtive). Data table Data table them to retail stores. The company has three inventory items: and floor lamps. RLC uses a perpetual inventory...
-
question 1- You borrow a simple loan of SR 500,000, interest rate is 20%, it matures in one year. what's the yied to maturity? question 2- calculate_i for One-Year Discount Bond with price(p) =...
-
Taste of Muscat is a reputed chain of restaurants operating in Oman. Assume You are working as a management accountant for this restaurant chain which is specialized in all types of Arabic food. Your...

Study smarter with the SolutionInn App


