What is the DU of estrone, a female sex hormone? Use the number of carbons and oxygen?s
Question:
What is the DU of estrone, a female sex hormone? Use the number of carbons and oxygen?s in estrone to calculate the number of hydrogen?s it has.
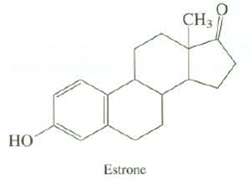
Transcribed Image Text:
НО Estrone 0 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The Du of estrone is ...View the full answer

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What is the Du Pont method for calculating return on investment? What is the advantage of this method?
-
What is the Du Pont Equation? And why is it useful?
-
The sex hormone estrone has been synthesized by a route that involves the following step. Identify the pericyclic reactions involved, and propose amechanism. CH CH Heat |CH30 CH0 Estrone methyl ether
-
Sketch the six graphs of the x- and y-components of position, velocity, and acceleration versus time for projectile motion with x 0 = y 0 = 0 and 0 < 0 < 90.
-
Outline the differences between extrinsic and intrinsic motivation, and explain how organisations can encourage intrinsic motivation.
-
Halm Skidoos Limited, a private company that began operations in 2014, always values its inventories at their current net realizable value. The company uses ASPE. Its annual inventory figure is...
-
Leaky process pumps. Quality (February 2008) presented a problem that actually occurred at a company that produces process pumps for a variety of industries. The company recently introduced a new...
-
Upscale hotels in the United States recently cut their prices by 20 percent in an effort to bolster dwindling occupancy rates among business travelers. A survey performed by a major research...
-
Would you expect the stock to increase or decrease after the ex-dividend date? Jeff Correct! Ashton Wyatt Continue Decrease How much of a decrease would you expect this will be
-
Cost data for Johnstone Manufacturing Company for the month ended March 31 are as follows: a. Prepare a cost of goods manufactured statement for March. b. Determine the cost of goods sold for March....
-
Amino acids such as alanine actually exist as species called zwitterions, with a positive charge on the nitrogen and a negative charge on the oxygen. Explain what effect you expect this to have on...
-
Explain whether each pair of models represents isomers or the same compound. (All represent compounds with the formula C7H16.) Draw structures for each compound represented by the models.
-
Sondgeroth Ltd. reports the following pretax income (loss) for both financial reporting purposes and tax purposes. The tax rates listed were all enacted by the beginning of 2020. Instructions a....
-
Your introduction needs to include the following. o Include a clear definition of unemployment and inflation and how and why they occur and rise in the economy. o Briefly provide your understanding...
-
Questions: 1. What strategies can be employed to foster a sense of inclusion and belonging within teams, and what are the potential benefits of doing so? 2. How can a team be successful? 3. What is...
-
Critical reflection involves closely examining events and experiences from different perspectives to inform future practice. In a few paragraphs, explain - Why educators should regularly reflect on...
-
What resources does the school or school district provide to teachers to promote diversity, equity, and inclusion? What are some of the strengths and shortcomings of the school's policies on...
-
Select FOUR companies listed on the UK Stock Exchange. Chose two companies from one industry sector and two other companies from another industry sector. By using the most recent three years'...
-
Make a choice and begin to map a strategy for how to accomplish the goal.
-
What is your assessment of the negotiations process, given what you have studied? What are your recommendations for Mr. Reed? You must justify your conclusions
-
When a particle is located a distance x feet from the origin, a force of x 2 + 2x pounds acts on it. How much work is done in moving it from x = 1 to x = 3?
-
When the 1HNMR spectrum of an alcohol is run in dimethyl sulfoxide (DMSO) solvent rather than in chloroform, exchange of the OH proton is slow and spinspin splitting is seen between the OH proton...
-
Give IUPAC names for the following compounds: (a) (b) (c) (d)
-
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (i) Na...
-
Long-term liabilities are shown in two places in the business firm's balance sheet depending upon when the long-term liabilities are scheduled for payment. True False
-
Julio is single with 1 withholding allowance. He earned $1,025.00 during the most recent semimonthly pay period. He needs to decide between contributing 3% and $30 to his 401(k) plan. If he chooses...
-
Acquirer firm plans to launch a takeover of Target firm. The manager of Acquirer indicates that the deal will increase the free cash flow of the combined business by $13.6m per year forever. The beta...

Study smarter with the SolutionInn App


