Question: Based on the following data, compare the effect of crystal size on solubility in water at 25C for (1) KC1 (see Example 17.7), a soluble
Based on the following data, compare the effect of crystal size on solubility in water at 25°C for (1) KC1 (see Example 17.7), a soluble inorganic salt, with that for (2) BaS04, an almost insoluble inorganic salt, and (3) sucrose, a very soluble organic compound.
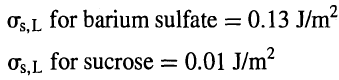
What conclusions can you draw from theresults?
for barium sulfate = 0.13 J/m2 for sucrose = 0.01 J/m? Os,L %3D
Step by Step Solution
3.27 Rating (171 Votes )
There are 3 Steps involved in it
From 1716 From a handbook Use SI units in 1 KCl Solubil... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (580).docx
120 KBs Word File


