Question: Below, atomic radius, crystal structure, electro negativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii
Below, atomic radius, crystal structure, electro negativity, and the most common valence are tabulated, for several elements; for those that are nonmetals, only atomic radii are indicated.
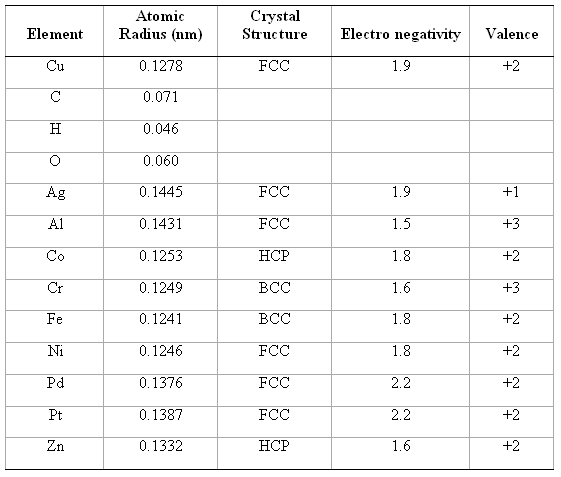
Which of these elements would you expect to form the following with copper?
(a) A substitutional solid solution having complete solubility
(b) A substitutional solid solution of incomplete solubility
(c) An interstitial solidsolution
Atomic Crystal Structure Element Radius (nm) Electro negativity Valence Cu 0.1278 FCC 1.9 +2 C 0.071 0.046 0.060 Ag 0.1445 FCC 1.9 +1 A1 0.1431 FCC 1.5 +3 Co 0.1253 1.8 +2 Cr 0.1249 1.6 +3 Fe 0.1241 1.8 +2 Ni 0.1246 FCC 1.8 +2 Pd 0.1376 FCC 2.2 +2 Pt 0.1387 FCC 2.2 +2 Zn 0.1332 1.6 +2
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
In this problem we are asked to cite which of the elements listed form with Cu the three possible so... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (94).docx
120 KBs Word File


