Show the structure of the carbocation that is observed when this compound is dissolved in superacid. CH3
Question:
Show the structure of the carbocation that is observed when this compound is dissolved in superacid.
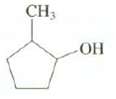
Transcribed Image Text:
CH3 ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (21 reviews)

Answered By

Rustia Melrod
I am a retired teacher with 6 years of experience teaching various science subjects to high school students and undergraduate students. This background enables me to be able to help tutor students who are struggling with the science of business component of their education. Teaching difficult subjects has definitely taught me patience. There is no greater joy for me than to patiently guide a student to the correct answer. When a student has that "aha!" moment, all my efforts are worth it.
The Common Core standards are a useful yardstick for measuring how well students are doing. My students consistently met or exceeded the Common Core standards for science. I believe in working with each student's individual learning styles to help them understand the material. If students were struggling with a concept, I would figure out a different way to teach or apply that concept. I was voted Teacher of the Year six times in my career. I also won an award for Innovative Teaching Style at the 2011 National Teaching Conference.
4.90+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the structure of a PLA with three inputs (C, B, A) and four outputs (O0, O1, O2, O3), with the outputs defined as follows: o,-ABC AB + AB O ABC ABC 02-C 03-AB + AB
-
Show the structure of the product you would expect to obtain by SN2 reaction of a cysteine residue with iodoacetic acid.
-
Show the structure of the polymer that result from heating the following di-epoxide anddiamine: Heat + H2N- -NH2
-
Figure 2 shows a 250 kg beam BC at the time instant when a 100 N horizontal force is applied to its end B for 10 s. a. Determine the tension in rods AB and CD at an arbitrary instant during the time...
-
How are the costs of the beginning work in process inventory treated differently under the weighted average and FIFO methods?
-
On January 1, 2017, Holland Corporation paid $8 per share to a group of Zeeland Corporation shareholders to acquire 60,000 shares of Zeeland's outstanding voting stock, representing a 60 percent...
-
What are the four common methods for binning numerical predictors? Which of these are preferred? Use the following data set for Exercises 2830: 111337
-
On January 1, 2011, Lennon Corporation acquires 100% of Ono Inc. for $220,000 in cash. The condensed balance sheets of the two corporations immediately following the acquisition are as follows....
-
From the lenders point of view, an ARM a. limits interest rate risk but potentially increases default risk b. limits default risk but potentially increases interest rate risk c. decreases both...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
The reaction of 3-iodo-2, 2-dimethylbutane with ethanol gives three elimination products in addition to two substitution products as shown in the following equation. Show all the steps in the...
-
How much is the reaction rate for these reactions increased or decreased if the concentration of hydroxide ion is doubled? If the concentrations of the both the alkyl chloride and hydroxide ion...
-
Steam is generated in the boiler of a cogeneration plant at 10 MPa and 450C at a steady rate of 5 kg/s. In normal operation, steam expands in a turbine to a pressure of 0.5 MPa and is then routed to...
-
In an air-pollution study performed at an experiment station, the following amount of suspended benzenesoluble organic matter (in micrograms per cubic meter) was obtained for eight different samples...
-
The figure shows a sketch of the curve with equation y = f(x). The curve passes through the points (0, 3) and (4, 0) and touches the x-axis at the point (1, 0). On separate diagrams, sketch the...
-
An object is placed \(200 \mathrm{~mm}\) from a diverging thin lens that has a focal length of \(-500 \mathrm{~mm}\). What are (a) the image distance and \((b)\) the magnification? (c) Draw a...
-
In a study of warp breakage during the weaving of fabric (Technometrics [1982]: 63), 100 pieces of yarn were tested. The number of cycles of strain to breakage was recorded for each yarn sample. The...
-
Many consider family-owned businesses the backbone of American business. Mei Mei translates from Chinese to little sister in English, and its name aptly represents a family business of three...
-
To evaluate the degree of responsibility in collaborating in other peoples actions
-
As economic conditions change, how do banks adjust their asset portfolio?
-
Solve each equation. -57 = 3.x
-
Fill in the missing reagents a?h in the following scheme: CO2Et CH3 CO2Et 2. f 1. c 2. d 1. a 2. b 1. g 1. e 2. h CO2Et
-
How would you prepare the following compounds fromCyclohexanone? la) (b) o C6H5CH, CHC&H5 CH2CH2CN (c) (d) CH2CH=CH2 CO2Et
-
Leucine, one of the twenty amino acids found in proteins, is metabolized by a pathway that includes the following step. Propose amechanism. "02C "C H3C SCOA SCOA 3-Hydroxy-3-methyl- glutaryl CoA...
-
Lakeland Inc. manufactured 2,500 units during the month of March. They incurred direct materials cost of $58,000 and overhead costs of $40,000. If their per-unit prime cost was $32.00 per unit, how...
-
Present Value Computations Using the present value tables, solve the following. ( Click here to access the PV and FV tables to use with this problem. ) Round your answers to two decimal places....
-
A company provided the following data: Sales $887,000 Variable costs $546,800 Fixed costs $310,000 Expected production and sales in units 36,000 What is the break-even point in sales dollars? Please...

Study smarter with the SolutionInn App


