In the production of cumene from propylene, the following elementary, vapor-phase, irreversible reaction takes place: The reaction
Question:
In the production of cumene from propylene, the following elementary, vapor-phase, irreversible reaction takes place: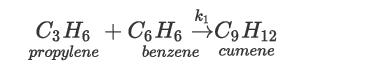
The reaction rate is given by![r = kCpc mol/(g x 104 exp cat)/s and k = 3.5 -12, 530 T[K]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/4/1/735654b6c479626a1699441734585.jpg)
The feed to a fluidized bed reactor consists of an equal ratio of benzene and propylene. The reaction takes place in a fluidized bed reactor operating at 300°C and 3 MPa pressure. For this problem, the fluidized bed may be assumed to be a constant-temperature CSTR reactor.
At design conditions, you may assume that side reactions do not take place to any great extent and the conversion is 68%.
It is desired to scale up production by 25% and all flows to the reactor will increase by 25% at the same feed concentration. Determine the following:
1. What is the single-pass conversion if the process conditions and amount of catalyst remain unchanged?
2. What percentage change in catalyst would be required to achieve the scale-up assuming that the pressure, temperature, and conversion were held constant?
3. Estimate how much the temperature would have to be changed (without changes in catalyst amount, operating pressure, or conversion) to achieve the desired scale-up.
4. By how much would the pressure have to be changed (without changes in catalyst amount, operating temperature, or conversion) to achieve the desired scale-up?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





