The pressure P, volume V, and temperature T of a van der Waals gas with n molecules
Question:
The pressure P, volume V, and temperature T of a van der Waals gas with n molecules (n constant) are related by the equation
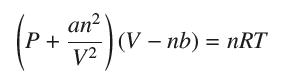
where a, b, and R are constant. Calculate ∂P/∂T and ∂V/∂P.
Transcribed Image Text:
(P + 1 ) (V V2 (V-nb) = nRT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
By Eq 7 Let F be the following function We compute the parti...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
In physical chemistry, it is shown that the pressure P of a gas is related to the volume V and temperature T by van der Waals equation: where a, b, n, and R are constants. The critical temperature T...
-
Through a combination of legal study and a targeted examination of TikTok\'s business and associated risks, your team can provide a comprehensive knowledge of the opportunities and problems...
-
Lets look at another way to compute phase diagrams from an equation of state without using departure functions but still requiring a numerical solution. The van der Waals equation of state can be...
-
North American Badgers (Taxidea taxus) occur throughout the western United States and Great Plains of North America, with the geographic range extending east to central Ohio (Messick, 1987; Whitaker...
-
A sedimentation process is to be used to separate pulverized coal from slate. A suspension of finely divided particles of galena (lead sulfide SG = 7.44) in water is prepared. The overall specific...
-
Explain why HF is a weak acid, whereas HCl, HBr, and HI are all strong acids.
-
What is the normal balance of the Treasury Stock account?
-
For 2008, Orchard Corporation reported after-tax net income of $5,800,000. During the year, the number of shares of stock outstanding remained constant at 10,000 of $100 par, 9 percent preferred...
-
Gaya Coyles dream is to buy her very own apartment unit in Syd. After a 6-month search, shes ecstatic to finally find the perfect unit, a brand new 2-bedroom unit located in Bankstown. She made an...
-
When x, y, and z are related by an equation F(x, y, z) = 0, we sometimes write (z/x) y in place of z/x to indicate that in the differentiation, z is treated as a function of x with y held constant...
-
For all x > 0, there is a unique value y = r(x) that solves the equation y 3 + 4xy = 16. (a) Show that dy/dx = 4y/(3y + 4x). (b) Let g(x) = f(x, r(x)), where f(x, y) is a function satisfying...
-
Suppose a 60.0-kg woman floats in freshwater with 97.0% of her volume submerged when her lungs are full of air. What is her average density? Strategy We can find the womans density by solving the...
-
Propose how these mechanisms can be used to build a strategic business partnership, close the gap between management / leadership and employees while building a cohesive culture that adds value,...
-
1.What risks does the company face? 2. What is role for ERM at Swissgrid or most any company? 3. What risk management processes has Meyer installed at Swissgrid? Assess their strengths and...
-
Elizabeth's Country Wares How many workers does Elizabeth have and what does each of them do? What type of work does Elizabeth do for the CP product line? How long does it take to do the underglazing...
-
Do you support the policy of not allowing some Chinese nationals to attend graduate school in the United States because of national security concerns?
-
Using your product or service name or category, do a search using the following phrase: Find a (insert the name of your product or service here...) near me. For instance, using my Mobile Notary...
-
Is it possible for a third-degree polynomial with rational coefficients to have no real zeros? Why or why not?
-
Show that if A is any m n matrix, then Im A = A and AIn = A.
-
According to the Girl Scouts of America, in March 2006, 9% of all Girl Scout cookies sold are shortbread/trefoils. If a box of Girl Scout cookies is selected at random, what is the probability that...
-
A golf ball is selected at random from a container. If the container has 9 white balls, 8 green balls, and 3 orange balls, find the probability of each event. The golf ball is white or green.
-
A golf ball is selected at random from a container. If the container has 9 white balls, 8 green balls, and 3 orange balls, find the probability of each event. The golf ball is white or orange.
-
American Food Services, Incorporated leased a packaging machine from Barton and Barton Corporation. Barton and Barton completed construction of the machine on January 1 , 2 0 2 4 . The lease...
-
Which of the following statements is true? Financial measures tend to be lag indicators that report on the results of past actions. LA profit center is responsible for generating revenue, but it is...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 8 0 , 0 0 0 Daks each year at a selling price of $ 5 6 per unit. The company s unit costs at this level of...

Study smarter with the SolutionInn App


