0.10 mol of gas undergoes the process 1 2 shown in Figure P 16.59. a. What...
Question:
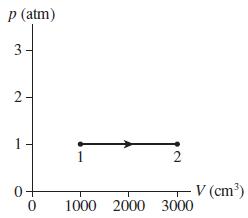
0.10 mol of gas undergoes the process 1 → 2 shown in Figure P 16.59.
a. What are temperatures T1 and T2 (in °C)?
b. What type of process is this?
c. The gas undergoes an isothermal compression from point 2 until the volume is restored to the value it had at point 1. What is the final pressure of the gas?
Transcribed Image Text:
p (atm) 3- 1 V (cm³) 1000 2000 3000 2. 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Model Assume the gas is an ideal gas Solve a We can find the temperatures di...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
0.0050 mol of gas undergoes the process 1?? 2 ?? 3 shown in Figure P16.38.? What are (a) Temperature T 1 , (b) Pressure p 2 , (c) Volume V 3 ? p (atm) P2 - T = 2926 K 3 T3 = 2438 K 1 0+ V (cm) V3...
-
0.0040 mol of gas undergoes the process shown in FIGURE EX18.35. a. What type of process is this? b. What are the initial and final temperatures in °C? p (atm) 3- 2- V (cm) 300 0+ 100 200 FIGURE...
-
0.020 mol of gas undergoes the process shown in FIGURE EX18.37. a. What type of process is this? b. What is the final temperature in °C? c. What is the final volume V 2 ? p (atm) 3- 2- 1- V (cm)...
-
Given are five observations for two variables, x and y. a. Develop a scatter diagram for these data. b. What does the scatter diagram developed in part (a) indicate about the relationship between the...
-
In the production of ethanol, the feedstock high in sugar content is first converted to sugar, and the sugar (glucose) is fermented into ethanol through the reaction C6H12O6 2 C2H5OH + 2 CO2....
-
Simpson Company, a calendar year taxpayer, acquires an apartment building on March 22, 2021 for $900,000. What is the maximum cost recovery deduction it may take for 2021?
-
Rectify the following errors: (a) Cash payment of ` 1000 to Dina was credited to his account in the ledger. (b) Cash receipt of ` 300 from Ravi was posted to the credit of Prasad. (c) Sales return of...
-
The following trial balance has been extracted from Carol Ltd as at 30 April 2012: Additional information: 1. Stock at 30 April 2012 was valued at 140,000. 2. Depreciation for the year of 28,000 is...
-
A tornado destroys your office building on 6/1/2019. Here are the facts $100,000 Historical cost of office building $40,000 depreciation taken on office building $250,000 fair market value before...
-
The statement of cash flows for Baldwin Company shows what happens in the Cash account during the year. It can be seen as a summary of the sources and uses of cash (sources of cash are added, uses of...
-
A gas cylinder with a tight-fitting, movable piston contains 200 cm 3 of air at 1.0 atm. It floats on the surface of a swimming pool filled with 15C water. The cylinder is then pulled slowly...
-
Compare and contrast software engineering with other engineering disciplines.
-
Stayman, Inc., manufactures products F, G, and H from a joint process. Additional information is as follows: mp5 Units produced Joint cost Sales value at split-off Sales value if processed further...
-
Task 2 In addition to the report produced for Task 1, the SMT have asked that you produce a short presentation, (minimum of 2 slides per bullet point), to help ensure that employees handle, store and...
-
Real solutions for x 2 = 5 ( x + 3 6 0 ) ?
-
1) Two-stage compressor with irreversibilities = You need to build a two-stage compression system with intercooling to increase the pressure of Argon (monatomic gas, constant specific heat) from pi...
-
A tightrope is connected at each end to a vertical tree trunk at a height of 1.57 meter above the ground. The two trees are located a distance 5.00 meters apart. At the midpoint of the tightrope, a...
-
A missing order occurs when a maximum of the two-slit diffraction pattern lines up with the minimum of the single slit diffraction pattern. Adjust the parameters of the simulation to create a...
-
Express each of the following ratios in its lowest terms. 11.7 : 7.8 : 3.9
-
The activities listed in lines 2125 serve primarily as examples of A) Underappreciated dangers B) Intolerable risks C) Medical priorities D) Policy failures
-
Hookes law describes an ideal spring. Many real springs are better described by the restoring force (F Sp ) s = -ks - q(s) 3 , where q is a constant. Consider a spring with k = 250 N/m and q = 800...
-
Hookes law describes an ideal spring. Many real springs are better described by the restoring force (F Sp ) s = -ks - q(s) 3 , where q is a constant. Consider a spring with k = 250 N/m and q = 800...
-
The force acting on a particle is F x = F 0 e-x/L . How much work does this force do as the particle moves along the x-axis from x = 0 to x = L?
-
Problem Set Time Value of Money 1. In 10 years, what is the value of $100 invested today at an interest rate of 8% per year, compounded annually? 2. In 10 years, what is the value of $100 invested...
-
The Blending Department of Luongo Company has the following cost and production data for the month of April. Costs: Work in process, April 1 Direct materials: 100% complete $120,000 Conversion costs:...
-
Q3 plz answer correctly and check work Builtrite's upper management has been comparing their books to industry standards and came up with the following question: Why is our operating profit margin...

Study smarter with the SolutionInn App


