a. Draw the skeletal formula of pentane, a straight chain hydrocarbon with a molecular formula of C
Question:
a. Draw the skeletal formula of pentane, a straight chain hydrocarbon with a molecular formula of C5H12.
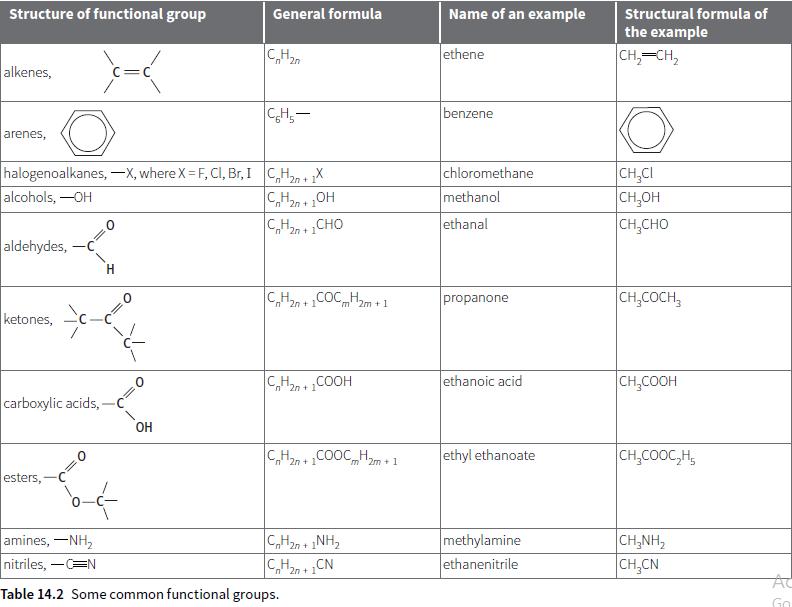
b. Draw the structural formulae of the molecules shown in Figure 14.2, parts d, e and f.
Transcribed Image Text:
Structure of functional group General formula Name of an example Structural formula of the example C,H2 ethene CH,-CH, n "2n alkenes, C=C CH,- benzene arenes, halogenoalkanes, –X, where X = F, CI, Br, I C,H, + X alcohols, -OH chloromethane methanol CH,CI CH,OH CH,CHO "2n 2n+ C,H2n + 1CHO ethanal aldehydes, C,H2n +COCHm + 1 CH,COCH, propanone "m' ketones, C,H2n + COOH ethanoic acid CH,COOH carboxylic acids, OH C,Han +COOC Hm+1 ethyl ethanoate CH,COOC,H, m' 2m + 1 esters,- amines, -NH, nitriles, -CN methylamine ethanenitrile CH,NH, 2n + C,H20 + CN CH,CN AC Table 14.2 Some common functional groups. Go
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
a Draw the skeletal formulae of: i. Pentan-2-one ii. Pentan-3-one iii. Pentanal. b. Describe the results you would expect to see if pentan-3-one and pentanal were separately treated with Tollens...
-
a. Draw the displayed formula and the skeletal formula of cyclopentane. b. What is the general formula of cycloalkanes? c. Give two differences between a molecule of cyclopentane and a molecule of...
-
Draw two different structural formulas based on the molecular formula C2H6O. Is the fact that you can have more than one compound with the same molecular formula consistent with Dalton's atomic...
-
XY is a pharmaceutical company with the head-quarter in the United States. However, its cash flow depends only on sales in Japan. It receives a cash flow of Yen 20 billion with certainty in t=1. The...
-
This problem is based on information taken from The Rating Guide to Life in Americas Fifty States, by G. S. Thomas. A random sample of n1 = 288 voters registered in the state of California showed...
-
Newtech Corp. is going to adopt a new chip-testing device that can greatly improve its production efficiency. Do you think the lead engineer can profit from purchasing the firm's stock before the...
-
In 1991, hikers in the Alps discovered the remains of a man who had been trapped in a glacier (Fig. P30.67). Carbon dating revealed the 14 6 C/ 12 6 C ratio in the remains to be 6.8x10 13 . How old...
-
The SS Minnow is lost at sea in a deep fog. Moving on a bearing of 107°, the skipper sees a light at a bearing of 60°. The same light reappears through the fog after the skipper has sailed...
-
Please answer question #8, SHOWING ALL WORK ACCORDINGLY. Thank you! Novak Corp. was organized on January 1, 2021. During its first year the corporation issued 2,100 shares of $50 par value preferred...
-
Bob Stevens is taking Managerial Accounting at State University next term and asked his friend, Summer Adams, who has already taken the course, to explain its focus Are we going to learn more about...
-
A chemist was investigating the best way to produce 1,2-dichloroethane. He devised two methods, I and II, of doing this. I . He reacted ethane with chlorine in the presence of UV light by the...
-
Draw a 3D formula for: a. Propane b. Propene.
-
a) Express these orders as a 1 4 matrix. b) Use matrix multiplication to determine the amount of each ingredient needed to fill the days order. In Exercise, use the information given in Exercise 53....
-
Use the information below to answer the next question. Below are different graphs that could represent the magnitude of an Electric Field from a source. Teza E Distance E 4 Tza E Taza 2 Distance 5 3...
-
Factor out the GCF: 36c5 +54c8
-
Demonstrate that a circle with a radius of r has a circumference of 2 pi ( r ) . HINT: Begin by examining the equation for the upper semicircle, utilize the arc length formula, and then double the...
-
Graph the function f(x) = 3.x - 7.
-
Vine plc. produces a single product. The following information on inventory, purchases, and sales are available for the month of January 2018. DATE TRANSACTION NUMBER OF UNITS UNIT COST...
-
[This is a variation of E 12?12 modified to focus on the fair value option.]Colah Company purchased $1 million of Jackson, Inc., 5% bonds at their face amount on July 1, 2021, with interest paid...
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
A vessel containing a liquid is opened inside an evacuated chamber. Will you see a liquidgas interface if the volume of the initially evacuated chamber is a. Less than the critical volume, b. A...
-
Calculate S R for the reaction H 2 (g) + Cl 2 (g) 2HCl (g) at 870. K. Omit terms in the temperature dependent heat capacities higher than T 2 /K 2 .
-
Use the result of Problem P3.10 to derive a formula for (CV /V ) T for a gas that obeys the RedlichKwong equation of state, RT 1 a Vm - b VT VVm + b)' T VVm P:
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...
-
Assume that gasoline costs $ 3 . 2 0 per gallon and you plan to keep either car for six years. How many miles per year would you need to drive to make the decision to buy the hybrid worthwhile,...

Study smarter with the SolutionInn App



