a. What is the oxidation number of the transition metal in each of the following complexes? i.
Question:
a. What is the oxidation number of the transition metal in each of the following complexes?
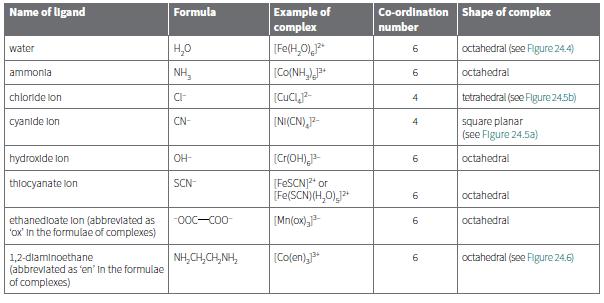
i. [Co(NH3)6]3+
ii. [Ni(CN)4]2–
iii. [Cr(OH)6]3–
iv. [Co(en)3]3+
v. Cu(OH)2(H2O)4
b. EDTA4– ions can act as ligands. A single EDTA4–ion can form six co-ordinate bonds to a central transition metal ion to form an octahedral complex. It is called a hexadentate ligand. Give the formula of such a complex formed between Ni2+and EDTA4–.
c. Which ligands in Table 24.4 are bidentate?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





