The lattice energy of magnesium bromide, MgBr 2 , can be calculated using the enthalpy changes shown
Question:
The lattice energy of magnesium bromide, MgBr2, can be calculated using the enthalpy changes shown in the table.
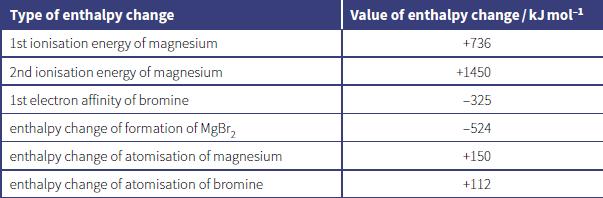
a. State the meaning of the terms:
i. Lattice energy
ii. 2nd ionisation energy.
b. Draw and label a Born–Haber cycle to calculate the lattice energy of magnesium bromide.
c. Calculate the lattice energy of magnesium bromide.
Transcribed Image Text:
Type of enthalpy change Value of enthalpy change / kJ molH 1st ionisation energy of magnesium +736 2nd ionisation energy of magnesium +1450 1st electron affinity of bromine -325 enthalpy change of formation of MgBr, -524 enthalpy change of atomisation of magnesium +150 enthalpy change of atomisation of bromine +112
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
a iEnergy change when one mole of an ionic compound forms fr...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
A stock's beta coefficient can be calculated using the following equation: B- = ^2 Vi, a. Write a user-defined function that can calculate the beta coefficient. The arguments to the function should...
-
The lattice energy of an ionic solid such as NaCl is the enthalpy change H° for the process in which the solid changes to ions. For example, NaCl(s) Na+(g) + Cl(g) H = 786 kJ/mol Assume that the...
-
The lattice energy of sodium chloride can be calculated using the following enthalpy changes: Enthalpy change of formation of sodium chloride Enthalpy changes of atomisation of sodium and chlorine ...
-
SG Company acquired 80% of Popsters Company on January 1, 2019, when the stockholders equity of Popsters consisted of: Ordinary shares, P100 par P500,000 Paid in capital in excess of par 400,000...
-
What is the meaning of the term statistical inference? What
-
A baseball game consists of plays that can be described as follows: Play Description No advance An out where no runners advance. This includes strikeouts, pop ups, short flies, and the like....
-
PR 15-2 When preparing interim reports, does an entity need to use the same method to value inventory that they use at the annual report date? What options are accepted?
-
The balance sheet accounts of Detroit Machinery, Inc., had the following balances on October 31, 20X0: Following is a summary of the transactions that occurred during November: a. Collections of...
-
1. A Roth IRA distribution will be reported on line 4a, but will not be included on line 4b of 1040. 2. because a qualified Roth iIRA (code Q) is not taxable, you do not have to report the...
-
Gaffel Company produces two types of calculators: scientific and business. Both products pass through two producing departments. The business calculator is by far the more popular. The following...
-
a. Write equations to represent: i. The 1st ionisation energy of caesium ii. The 3rd ionisation energy of aluminium iii. The enthalpy change of formation of calcium oxide iv. The enthalpy change of...
-
a. Draw a fully labelled BornHaber cycle for potassium bromide, naming each step. b. State the name of the enthalpy changes represented by the following equations: i. I 2 (s) I(g) ii. N(g) + e N ...
-
A building has a heat loss of 100 MJ/hr at design conditions at the geographical location where you reside. Calculate fuel consumed over the heating season at the geographical location where you...
-
You must select an orifice meter for measuring the flow rate of an organic liquid ( $\mathrm{SG}=0.8$, $\mu=15 \mathrm{cP}$ ) in a $4 \mathrm{in}$. sch 40 pipe. The maximum flow rate anticipated is...
-
A team of designers was given the task of reducing the defect rate in the manufacture of a certain printed circuit board. The team decided to reconfigure the cooling system. A total of 973 boards...
-
Level 98%: x1 = 49, n1 = 74, x2 = 62, n2 = 153 In Exercises 712, construct the confidence interval for the difference p1 p2 for the given level and values of x1, n1, x2, and n2.
-
Let X be a continuous random variable with the following PDF Find the MGF of X, M X (s). fx(x) = +) == 12e-1|2|1 e-A/).
-
The number of hours spent studying per day by a sample of 28 students In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 2 8 7 2 261 82 35 37 25 20 73 83...
-
Jansen Companys general ledger showed a checking account balance of $23,820 at the end of May 2021. The May 31 cash receipts of $2,340, included in the general ledger balance, were placed in the...
-
Identify Thank You mission, strategy and core competencies. Identify strategy changes that have taken place at Thank You since its founding in 2008. Your answer must in text references and must be...
-
The initiation step for radical addition of HBr is highly endothermic: (a) Explain how this step can be thermodynamically favorable at high temperature even though it is endothermic. (b) Explain why...
-
Between 0°C and 100°C, the heat capacity of Hg(l) is given by Calculate ÎH and ÎS if 2.25 moles of Hg(l) is raised in temperature from 0.00° to 88.0°C at constant P....
-
Draw all resonance structures for each of the following radicals: (a) (b) (c) (d) (e)
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


