The melting points of the derivatives of the reaction between 2,4-DNPH and various aldehydes and ketones are
Question:
The melting points of the derivatives of the reaction between 2,4-DNPH and various aldehydes and ketones are shown in the table.
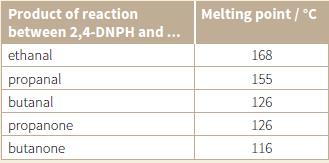
a. What would be observed when each of the carbonyl compounds in the table is mixed with 2,4-DNPH?
b. A derivative was formed between 2,4-DNPH and an unknown carbonyl compound.
i. The melting point of the derivative was 126 °C. What does this result tell you?
ii. The unknown carbonyl compound formed an orange precipitate when warmed with Fehling’s solution. Name the unknown compound.
iii. Describe and explain the different results obtained when the compound named in part b ii is warmed with Tollens’ reagent in a test tube and then the same test is performed on butanone.
c. Write a half-equation to show silver ions acting as an oxidising agent in a positive test for an aldehyde.
d. Write a half-equation to show copper(II) ions acting as an oxidising agent in a positive test for an aldehyde.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





