se the cell voltage method described in Worked examples 6 and 7 above to answer question 21,
Question:
se the cell voltage method described in Worked examples 6 and 7 above to answer question 21, parts a to d.
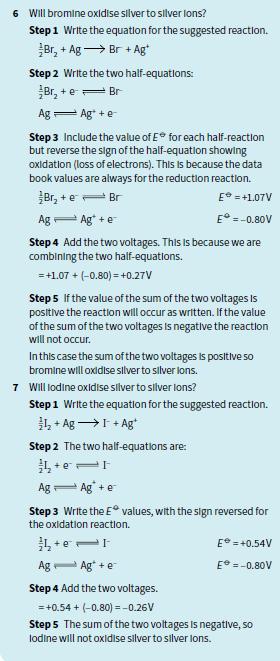
Data From Question 21:
Use the data in Appendix 2 (page 474) to predict whether or not the following reactions are feasible. If a reaction does occur, write a balanced equation for it.
a Can MnO4 – ions oxidise Cl– ions to Cl2 in acidic conditions?
b Can MnO4– ions oxidise F– ions to F2 in acidic conditions?
c Can H+ ions oxidise V2+ ions to V3+ ions?
d Can H+ ions oxidise Fe2+ ions to Fe3+ ions?
Data From Appendix 2:

6 Will bromine oxldise silver to silver lons? Step 1 Write the equation for the suggested reaction. Br, + Ag → Br + Ag Step 2 Write the two half-equations: Br, + e= Br Ag 一Ag +e Step 3 Include the value of E* for each half-reaction but reverse the sign of the half-equation showing oxidation (loss of electrons). This Is because the data book values are always for the reduction reaction. Br, + e- Br E = +1.07V Ag Ag* +e E° =-0.80V Step 4 Add the two voltages. This Is because we are combining the two half-equations. =+1.07 + (-0.80) = +0.27V Step 5 lf the value of the sum of the two voltages Is positive the reaction will occur as written. If the value of the sum of the two voltages is negative the reaction will not occur. Inthis case the sum of the two voltages is positive so bromine will oxidise slver to slver lons. 7 Will lodine oxidise silver to silver lons? Step1 Write the equation for the suggested reaction. 弘+ Ag→I+Ag Step 2 The two half-equations are: + e Ag- Ag +e Step 3 Write the E values, with the sign reversed for the oxidation reaction. E = +0.54V Ag- Ag* +e E =-0.80V Step 4 Add the two voltages. = +0.54 + (-0.80) =-0.26V Step 5 The sum of the two voltages Is negative, so lodine will not oxidise silver to silver lons.
Step by Step Answer:
To determine whether the given reactions are feasible we can use the cell voltage method This involv...View the full answer



Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Sciences questions
-
Examples 2 and 6 described a study about therapeutic touch (TT). A second run of the same experiment in the study used 13 TT practitioners who had to predict the correct hand in each of 10 trials. a....
-
A silver oxidezinc cell maintains a fairly constant voltage during discharge (1.60 V). The button form of this cell is used in watches, hearing aids, and other electronic devices. The half-reactions...
-
In Exercises 7 and 8 of Chapter 4 you worked with data on sales for a line of skiwear that is produced by HeathCo Industries. Barbara Lynch, product manager for the skiwear, has the responsibility of...
-
Using the information from 2-16, create a cash flow statement for 2013. In 2-16 Bristle Brush-Off Corporation: Income Statements for Years Ended December 31 ($000s) 2013 2012 $7,950 5,100 350 750...
-
Let z be a random variable with a standard normal distribution. Find the indicated probability and shade the corresponding area under the standard normal curve. P( 1.78 z 1.23)
-
Nadia Algar is the overworked IT resource person for her department. In the next round of computer purchases, she is determined to recommend a vendor who does a better job of documenting possible...
-
Project success is defined as adherence to budget, schedule, functionality (performance), and client satisfaction. Under these criteria, cite evidence that suggests the Sochi Olympics project was a...
-
Open the Multiplication Solution.sln file contained in the VB2017\Chap05\Multiplication Solution folder. Code the application to display a multiplication table similar to the one shown in Figure...
-
Conclusion - Walmart (as of January 31, 2021) Based on your analysis of the company (everything youve evaluated up to this point) , provide recommendations for whether: Creditors should loan money to...
-
Carbon Dioxide has a critical temperature of 304.13 K, critical pressure of 7377.3 kPa and critical volume of 9.41 x 105 m mol 1, By using the Van der Waals Equation of State, calculate the...
-
Suggest a suitable reagent that can carry out each of the following oxidations or reductions. Use the data in Appendix 2 to help you. a. The reduction of Zn 2+ ions to Zn. b. The oxidation of Br ...
-
In each of the chemical reactions a to c: i. Which species gains electrons? ii. Which species loses electrons? iii. Which species is the oxidising agent? iv. Which species is the reducing agent? a....
-
Which of the following is not true for a process costing system? a. Cost accumulation is by job. b. The cost transfer is at the end of the accounting period. c. It is used by those manufacturing...
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
At the end of the year, Breyer Associates had a credit balance in its allowance for uncollectible accounts of $12,000 before adjustment. The balance in Breyers gross accounts receivable is $600,000....
-
During the month, services performed for customers on account amounted to $7,500 and collections from customers in payment of their accounts totaled $6,000. At the end of the month, the Accounts...
-
A sample of Na2SO4(s) is dissolved in 225 g of water at 298 K such that the solution is 0.325 molar in Na 2 SO 4 . A temperature rise of 0.146C is observed. The calorimeter constant is 330. J K 1 ....
-
Assign a name for each of the following compounds. a. b. c.
-
a. Using the relationships derived in Example Problem 7.1 and the values of the critical constants for water from Table 7.2, calculate values for the van der Waals parameters a, b, and R from z c , T...
-
Present Value Computations Using the present value tables, solve the following. ( Click here to access the PV and FV tables to use with this problem. ) Round your answers to two decimal places....
-
A company provided the following data: Sales $887,000 Variable costs $546,800 Fixed costs $310,000 Expected production and sales in units 36,000 What is the break-even point in sales dollars? Please...
-
How to solve them..equation and explain ..please.. 1. Selected information from the companys financial records is presented below Equipment, December 31, 2013 $300,000 Equipment, December 31, 2014...

Study smarter with the SolutionInn App


