A thermodynamic property of a mixture is given by a. Develop expressions for the partial molar properties
Question:
A thermodynamic property of a mixture is given by
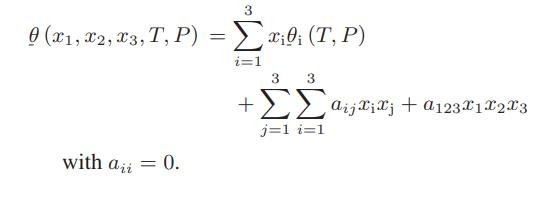
a. Develop expressions for the partial molar properties θ̅1, θ̅2, and θ̅3 as a function of the pure component molar properties, the mole fractions, and the parameters a12, a13, a12, and a123.
b. Obtain expressions for the infinite-dilution value of θ̅1 in solutions of varying concentrations of species 2 and 3. Repeat for θ̅2 in mixed solvent solutions of 1 and 3, and for θ̅3 in mixed solvent solutions of 1 and 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





