The following data are available for the mean ionic activity coefficients of these salts in water at
Question:
The following data are available for the mean ionic activity coefficients of these salts in water at 25°C.
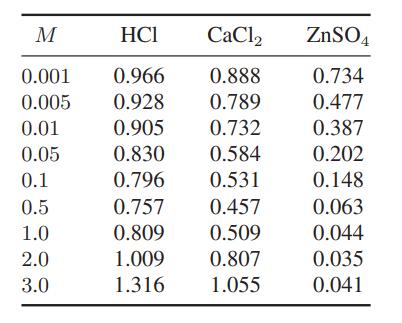
a. Fit these data as best you can using the equations in this chapter for the mean ionic activity coefficient.
b. Determine the activity coefficient of water in each of these solutions.
Transcribed Image Text:
M HCI 0.001 0.966 0.005 0.928 0.01 0.905 0.05 0.830 0.1 0.796 0.5 0.757 1.0 0.809 2.0 1.009 3.0 1.316 CaCl2 0.888 0.789 0.732 0.584 0.531 0.457 0.509 0.807 1.055 ZnSO4 0.734 0.477 0.387 0.202 0.148 0.063 0.044 0.035 0.041
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To fit the data for the mean ionic activity coefficients of the salts in water at 25Cwe can use the ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The following data are available for three companies at the end of their fiscal years: Required Determine the amounts indicated by question marks. Company A $ 600,000 Finished goods, January 1 Cost...
-
(a) Briefly explain what an embedded system is and why we need embedded system based solutions. (b) With the aid of simple functional block diagram, briefly explain how embedded systems work. (c)...
-
The following data are available for Haul-It-Away Truckers: 1. Compute the predetermined overhead rate for each of the two years, if based on (a) Direct labor hours, (b) Number of moving jobs, and...
-
Clark had brain surgery. Insurance will not pay for the surgery until the deductible of $1,000 is hit. Then Clark's coinsurance of 80% / 20% kicks in. Clark has an out-of-pocket maximum of $7,500....
-
Walesco Corporation has decided to discontinue an entire component of its business effective November 1, 2008. It hopes to sell the assets involved and convert the physical plant to other uses within...
-
Obtain copies of the American Institute of Architects (AIA) standard contract documents and compare them with other standard contracts (e.g., Engineers Joint Contract Documents Committee (EJCDC)...
-
Reasoning is a cognitive process in which people start with information and come to conclusions that go beyond that information. Deductive reasoning involves syllogisms and can result in definite...
-
Felter Company produced and sold 50,000 units of product and is operating at 70% of plant capacity. Unit information about its product is as follows: The company received a proposal from a foreign...
-
Question 21 Not yet answered Points out of 2.00 P Flag question The concept of the Thin Slice states that: Select one: O a. The first minute of communication is critical to persuasion o b. It's hard...
-
Chekov Company has two support departments, Human Resources and General Factory, and two producing departments, Fabricating and Assembly. Direct costs Normal activity: Required: Support Departments...
-
A thermodynamic property of a mixture is given by a. Develop expressions for the partial molar properties 1 , 2 , and 3 as a function of the pure component molar properties, the mole fractions,...
-
A gas stream at 310 K and 14 bar is to be compressed to 345 bar before transmission by underground pipeline. If the compression is carried out adiabatically and reversibly, determine the compressor...
-
The chart of accounts of Angels Delivery Service of Flin Flon includes the following: Cash, 111; Accounts Receivable, 112; Office Equipment, 121; Delivery Trucks, 122; Accounts Payable, 211; Alice...
-
Prove that the estimating equations in (11.13) are unbiased under MCAR, but are generally biased without the stringent MCAR assumption. (x) [y - f (xt;)] = 0, i=1 (11.13)
-
Refer to Figure 11.5: Which is the most expensive subcontract for this project? How much were the costs for the general contractor's crews for item 4? Figure 11.5 Division 1 2 3 4 5 6 7 Work Gen'l...
-
a. Using observations on the change in consumption \(D C_{t}=C_{t}-C_{t-1}\) and the change in income \(D Y_{t}=\) \(Y_{t}-Y_{t-1}\) from 1959Q3 to 2015Q4, obtained from the data file cons_inc,...
-
Water at \(20^{\circ} \mathrm{C}\) flows by gravity from a large reservoir at a high elevation to a smaller one through a 35-m-long, 5-cm-diameter cast iron piping system that includes four standard...
-
Customers arrive at a ferry ticket office at the rate of 14 per hour on Monday morn- ings. This can be described by a Poisson distribution. Selling the tickets and pro- viding general information...
-
Sketch the transfer characteristics of a p-channel enhancement-type MOSFET if VT = -5 V and k = 0.45 x 10-3A/V2.
-
In Exercises delete part of the domain so that the function that remains is one-to-one. Find the inverse function of the remaining function and give the domain of the inverse function. f(x) = 16x4 -3...
-
Which will be the strongest oxidizing agent under standard conditions (that is, all activities = 1): HNO 2 , Se, UO 2 2+ , Cl 2 , H 2 SO 3 , or MnO 2 ?
-
What is the difference between E and E for a redox reaction? Which one runs down to 0 when the complete cell comes to equilibrium?
-
(a) Use the Nernst equation to write the spontaneous chemical reaction that occurs in the cell in Demonstration 13-1. (b) If you use your fingers as a salt bridge in Demonstration 13-1, will your...
-
What is the difference between management's goals and the firm's goals? How can the two be in conflict? Provide examples of real world examples of when management and the owners of a company have...
-
Q) A stock price is currently $90. Over each of the next two 6-month periods, it is expected to go up by 10% or down by 10%. The risk-free interest rate is 8% per annum with continuous compounding....
-
A couple who borrow $80,000 for 30 years at 7.2%, compounded monthly, must make monthly payments of $543.03. (Round your answers to the nearest cent.) (a) Find their unpaid balance after 1 year. $...

Study smarter with the SolutionInn App


