Question: A distillation system with a still pot plus a column with one equilibrium stage is used to batch distill (1.0 mathrm{kmol}) of a (57.0 mathrm{~mol}
A distillation system with a still pot plus a column with one equilibrium stage is used to batch distill \(1.0 \mathrm{kmol}\) of a \(57.0 \mathrm{~mol} \%\) methanol and 43.0 \(\mathrm{mol} \%\) water feed. A total condenser is used. The final bottoms concentration is \(15.0 \mathrm{~mol} \%\) methanol. Pressure is \(101.3 \mathrm{kPa}\). Reflux is a saturated liquid, and \(\mathrm{L}_{0} / \mathrm{D}=1.85\). Find \(\mathrm{W}_{\text {final }}, \mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\). VLE data are in Table 2-8.
Table 2.8
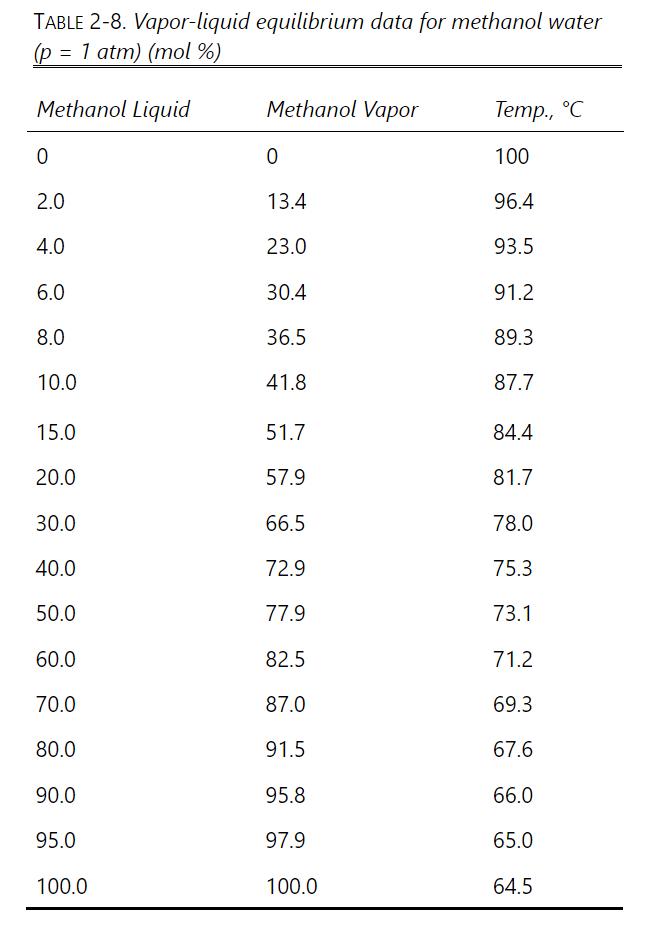
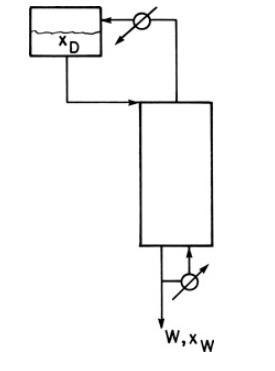
TABLE 2-8. Vapor-liquid equilibrium data for methanol water (p = 1 atm) (mol %) Methanol Liquid Methanol Vapor Temp., C 0 0 100 2.0 13.4 96.4 4.0 23.0 93.5 6.0 30.4 91.2 8.0 36.5 89.3 10.0 41.8 87.7 15.0 51.7 84.4 20.0 57.9 81.7 30.0 66.5 78.0 40.0 72.9 75.3 50.0 77.9 73.1 60.0 82.5 71.2 70.0 87.0 69.3 80.0 91.5 67.6 90.0 95.8 66.0 95.0 97.9 65.0 100.0 100.0 64.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


