Question: A simple batch still (one equilibrium stage) separates 100 moles of a 10.0 (mathrm{mol} %) methanol and (90.0 mathrm{~mol} %) water feed. The final bottoms
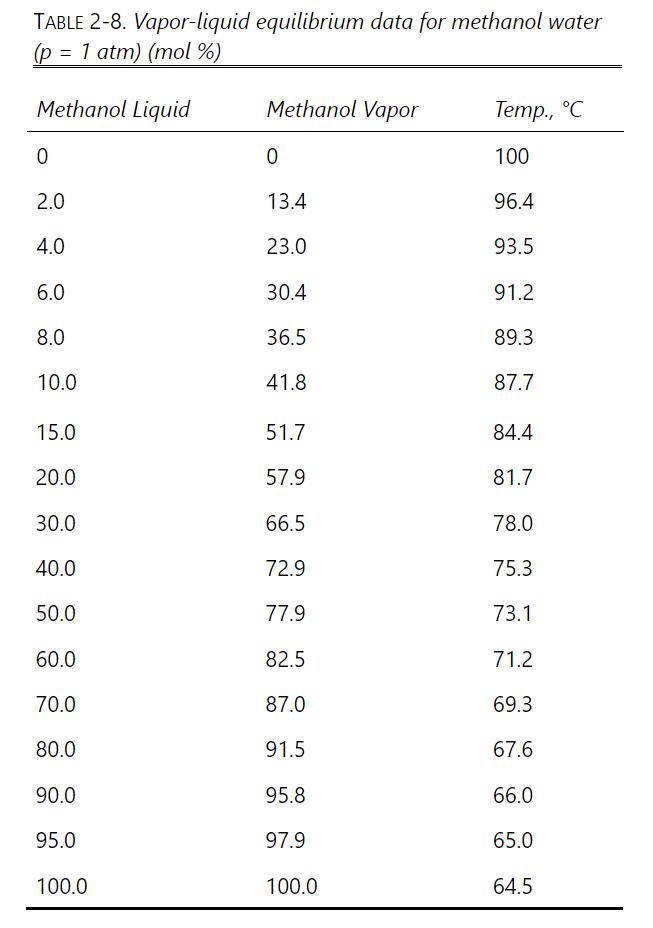
A simple batch still (one equilibrium stage) separates 100 moles of a 10.0 \(\mathrm{mol} \%\) methanol and \(90.0 \mathrm{~mol} \%\) water feed. The final bottoms concentration is \(1.0 \mathrm{~mol} \%\) methanol. VLE data are in Table 2-8. \(\mathrm{p}=1.0\) atm.
Table 2-8
a. Find \(\mathrm{W}_{\text {final }}\), distillate \(\mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\) using Simpson's rule with one part.
b. Find \(\mathrm{W}_{\text {final }}\), distillate \(\mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\) using the Gaussian quadrature formula.
c. Explain why the area calculated by Simpson's rule is too high.
TABLE 2-8. Vapor-liquid equilibrium data for methanol water (p = 1 atm) (mol %) Methanol Liquid Methanol Vapor Temp., C 0 0 100 2.0 13.4 96.4 4.0 23.0 93.5 6.0 30.4 91.2 8.0 36.5 89.3 10.0 41.8 87.7 15.0 51.7 84.4 20.0 57.9 81.7 30.0 66.5 78.0 40.0 72.9 75.3 50.0 77.9 73.1 60.0 82.5 71.2 70.0 87.0 69.3 80.0 91.5 67.6 90.0 95.8 66.0 95.0 97.9 65.0 100.0 100.0 64.5
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


