Question: We are doing RO experiments with a completely mixed laboratory unit. Experiment (A). With a very high stirrer speed so that there is no concentration
We are doing RO experiments with a completely mixed laboratory unit.
Experiment \(A\). With a very high stirrer speed so that there is no concentration polarization, we do an experiment with a feed that is \(0.77 \mathrm{wt} \%\) sodium chloride in water. Cut \(\theta^{\prime}\) (in mass units) is 0.22 , and measured inherent retention is \(\mathrm{R}^{\mathrm{o}}=0.9804\). Permeate pressure is \(1.1 \mathrm{~atm}, \mathrm{~T}=40.0^{\circ} \mathrm{C}\), and solvent flux is \(415.4 \mathrm{~g} /\left(\mathrm{m}^{2} \cdot \mathrm{s}\right)\). \(\mathrm{K}_{\text {solv }}^{\prime} / \mathrm{t}_{\mathrm{ms}}=33.29 \mathrm{~g} /\left(\mathrm{atm} \mathrm{m} \mathrm{m}^{2} \cdot \mathrm{s}\right)\). Data: Assume solutions have the same density as water \(=997 \mathrm{~kg} / \mathrm{m}^{3}\). Osmotic pressure correlations are given in Eq. (19-14i).
Eq. 19-14i
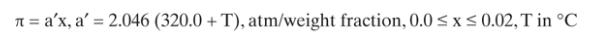 For experiment A, retentate pressure was not recorded. Calculate the value of retentate pressure that was used for this experiment. Remember to correct for \(\mathrm{T} eq 25^{\circ} \mathrm{C}\).
For experiment A, retentate pressure was not recorded. Calculate the value of retentate pressure that was used for this experiment. Remember to correct for \(\mathrm{T} eq 25^{\circ} \mathrm{C}\).
= a'x, a' = 2.046 (320.0 + T), atm/weight fraction, 0.0 x 0.02, T in C
Step by Step Solution
3.31 Rating (142 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


