(a) From data in Appendix 2A, derive a numerical form of the ClausiusClapeyron equation for methanol. (b)...
Question:
(a) From data in Appendix 2A, derive a numerical form of the Clausius–Clapeyron equation for methanol.
(b) Use the equation to plot the appropriate quantities that should give a straightline relation between vapor pressure and temperature.
(c) Estimate the vapor pressure of methanol at 0.0°C.
(d) Estimate the normal boiling point of methanol.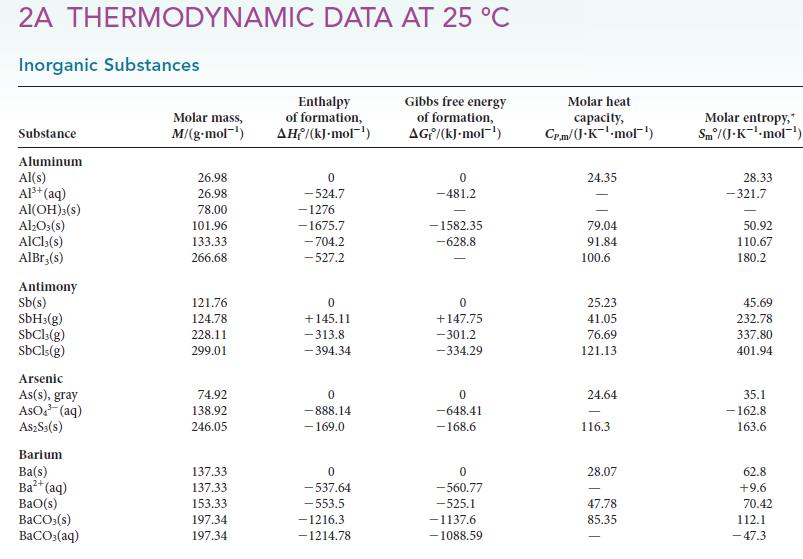
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO³(aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J-K-¹-mol¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a b The relationship ...View the full answer

Answered By

Seema kuldeep
although I don't have an experience of teaching in a particular institute, previously I was an expert on Chegg and I have used to teach my batch mates and also my juniors.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) From data in Appendix 2A and Table 4C.1, derive a numerical form of the ClausiusClapeyron equation for benzene. (b) Use the equation to plot the appropriate quantities that should give a...
-
In the process of distillation, a mixture of two (or more) volatile liquids is first heated to convert the volatile materials to the vapor state. Then the vapor is condensed, reforming the liquid....
-
The stock market consists of only 2 stocks, X and Y Their market capitalizations are $1.5bn and $8.5bn respectively. Their returns are uncorrelated. The mean return of X is 6% and that of Y is 11%....
-
Suppose that two linear equations are graphed on the same set of coordinate axes. Sketch what the graph might look like if the system has the given description. (a) The system has a single solution....
-
Staying up late to study, and having no stove to heat water, you use a 200-W heater from the lab to make coffee throughout the night. If 90% of the energy produced by the heater goes toward heating...
-
If the height and the base of a triangle are doubled, what happens to the area? Explain
-
Indicate how to present and analyze stockholders equity.
-
Chapman Pharmaceuticals, a large manufacturer of drugs, has this aggregate demand forecast for a liquid cold medicine. Demand 200 120 75 40 15 January February May une September October November...
-
Compute the NPV for Project M If the appropriate cost of capital is 7 percent. (Negative amount should be indicated by a minus sign. Do not round Intermediate calculations and round your final answer...
-
The American black bear (Ursus americanus) is one of eight bear species in the world. It is the smallest North American bear and the most common bear species on the planet. In 1969, Dr. Michael R....
-
A polymer sample of mass 0.20 g dissolved in 0.100 L of toluene, gives rise to an osmotic pressure of 6.3 Torr at 20C. What is the molar mass of the polymer?
-
The normal boiling point of ethyl acetate, CH 3 COOC 2 H 5 , used to remove nail polish, is 77.1C, and its vapor pressure at 16.2 C is 10.0 kPa. Calculate (a) The standard enthalpy of vaporization of...
-
Satire is a kind of glass, wherein beholders do generally discover everybodys face but their own. Criticize the following definitions in light of the eight rules for lexical definitions:
-
What is the logical ending point of a sequential game that starts at position (2,8) with player 1 moving first? Show your work. Player 1 Strategy B Strategy A Strategy A Player 2 Strategy B (3,4)...
-
Problem A-6 Income and Retained Earnings Statements Peanut Corporation is a private corporation using ASPE. At December 31, 2017, an analysis of the accounts and discussions with company officials...
-
8.5 Area Between Curves (dy) Calculus-Calculator Allowed Mastery Check #2 Name: Date: Period: For 1-2, find the area of the region bounded by the following curves. Show the integral set up with...
-
Your company has a travel policy that reimburses employees for the "ordinary and necessary" costs of business travel. Employees often mix a business trip with pleasure by either extending the time at...
-
Simulation A: 1 Diameter 600 mm 2 Focal Length 1800 mm 3 F/D Ratio 3 4 Eyepieces 30 m 5 Barlow? N 6 Celestial Sights M42 - M31 - M51 Simulation B: 1 Diameter 150 mm 2 Focal Length 1800 mm 3 F/D Ratio...
-
A steel tank holds 120 ft3 of water at atmospheric pressure (14. 7 lbs/in2 and 68.4F). The water subjected to a 100-fold increase in pressure. Determine the initial weight and final density of the...
-
Making use of the tables of atomic masses, find the velocity with which the products of the reaction B10 (n, ) Li7 come apart; the reaction proceeds via interaction of very slow neutrons with...
-
Propose a plausible mechanism for each of the following reactions: a. b. Br Br2 . [H,SO,]
-
Propose a plausible mechanism for the following process, called iodolactonization: I2
-
When 3-bromocyclopentene is treated with HBr, the observed product is a racemic mixture of trans-1,2-dibromocyclopentane. None of the corresponding cis-dibromide is observed. Propose a mechanism that...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


