Acetic acid is a weak acid commonly found in both laboratory and household, but to what extent
Question:
Acetic acid is a weak acid commonly found in both laboratory and household, but to what extent have its molecules actually been deprotonated? Calculate the pH and percentage deprotonation of CH3COOH molecules in 0.080 m CH3COOH(aq), given that Ka for acetic acid is 1.8 * 10–5.
ANTICIPATE Because the solution is that of an acid, expect pH
PLAN Following the procedure in Toolbox 6D.1, write the proton transfer equilibrium and construct the equilibrium table with concentrations in moles per liter.
What should you assume? You can make two assumptions, but they need to be verified at the end of the calculation.
(1) Deprotonation is so slight that the equilibrium concentration of the acid is approximately the same as its initial concentration.
(2) The autoprotolysis of water does not contribute significantly to the pH.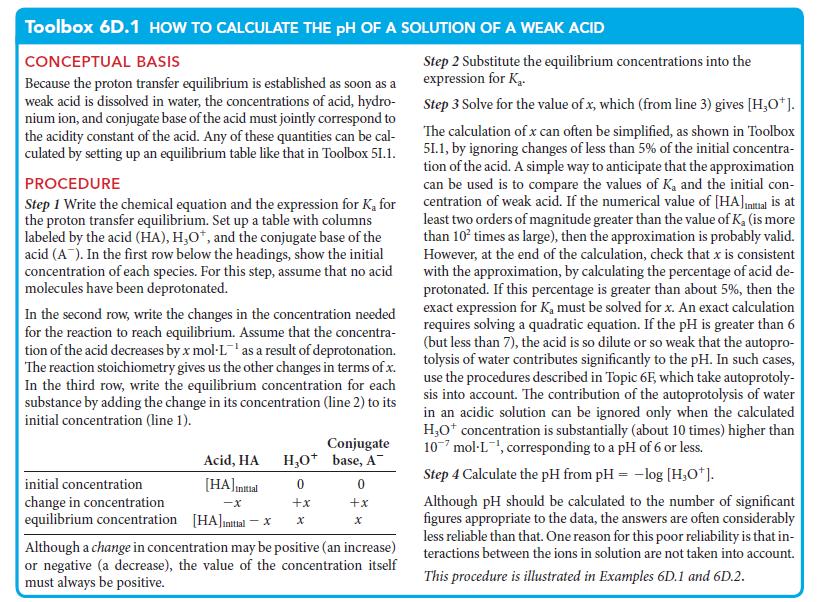
Toolbox 6D.1 HOW TO CALCULATE THE PH OF A SOLUTION OF A WEAK ACID CONCEPTUAL BASIS Because the proton transfer equilibrium is established as soon as a weak acid is dissolved in water, the concentrations of acid, hydro- nium ion, and conjugate base of the acid must jointly correspond to the acidity constant of the acid. Any of these quantities can be cal- culated by setting up an equilibrium table like that in Toolbox 51.1. PROCEDURE Step 1 Write the chemical equation and the expression for K, for the proton transfer equilibrium. Set up a table with columns labeled by the acid (HA), H₂O*, and the conjugate base of the acid (A). In the first row below the headings, show the initial concentration of each species. For this step, assume that no acid molecules have been deprotonated. In the second row, write the changes in the concentration needed for the reaction to reach equilibrium. Assume that the concentra- tion of the acid decreases by x mol-L¹ as a result of deprotonation. The reaction stoichiometry gives us the other changes in terms of x. In the third row, write the equilibrium concentration for each substance by adding the change in its concentration (line 2) to its initial concentration (line 1). initial concentration change in concentration equilibrium concentration Acid, HA [HA] initial -X [HA]initial - x H₂O+ 0 +x X Conjugate base, A™ 0 +x X Although a change in concentration may be positive (an increase) or negative (a decrease), the value of the concentration itself must always be positive. Step 2 Substitute the equilibrium concentrations into the expression for K₂. Step 3 Solve for the value of x, which (from line 3) gives [H₂O*]. The calculation of x can often be simplified, as shown in Toolbox 51.1, by ignoring changes of less than 5% of the initial concentra- tion of the acid. A simple way to anticipate that the approximation can be used is to compare the values of K, and the initial con- centration of weak acid. If the numerical value of [HA]Initial is at least two orders of magnitude greater than the value of K. (is more than 10² times as large), then the approximation is probably valid. However, at the end of the calculation, check that x is consistent with the approximation, by calculating the percentage of acid de- protonated. If this percentage is greater than about 5%, then the exact expression for K, must be solved for x. An exact calculation requires solving a quadratic equation. If the pH is greater than 6 (but less than 7), the acid is so dilute or so weak that the autopro- tolysis of water contributes significantly to the pH. In such cases, use the procedures described in Topic 6F, which take autoprotoly- sis into account. The contribution of the autoprotolysis of water in an acidic solution can be ignored only when the calculated H₂O* concentration is substantially (about 10 times) higher than 10 mol-L¹, corresponding to a pH of 6 or less. Step 4 Calculate the pH from pH = -log [H,0¹]. Although pH should be calculated to the number of significant figures appropriate to the data, the answers are often considerably less reliable than that. One reason for this poor reliability is that in- teractions between the ions in solution are not taken into account. This procedure is illustrated in Examples 6D.1 and 6D.2.
Step by Step Answer:
Step 1 The proton transfer equilibrium and the corresponding equilibrium table are CHCOOHaq HO1 H0aq ...View the full answer



Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Students also viewed these Sciences questions
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Although there are extensive tables available for the pK a of weak acids, you might be dealing with an unknown acid or a known acid at an unlisted temperature. You could then use a procedure like...
-
make a small case or use a situation/problem from real life. You will discuss this situation together with a discussion that works through a solution of your own to the problem posed. Problems or...
-
Variable costing and absorption costing the All-Fixed Company. It is the end of 2009. The All-Fixed Company began operations in January 2008. The company is so named because it has no variable costs....
-
Prove that uniform-cost search and breadth-first search with constant step costs are optimal when used with the GRAPH-SEARCH algorithm. Show a state space with constant step costs in which...
-
Clearly interpret the meaning of the slope coefficient.
-
Kaehler Enterprises earns 8% on an investment that pays back $90,000 at the end of each of the next 6 years. What is the amount Kaehler Enterprises invested to earn the 8% rate of return?
-
The records of Marshall Company include the following: Average total assets $4,450,000 Average total liabilities 2,170,000 Total revenue 4,130,000 Total expense (including income tax) 3,910,000...
-
An internet service provider has two connection lines for its customers. Eighty percent of all customers are connected through line I, and twenty percent are connected through line II. Line I has a...
-
Write (a) The chemical equation for the proton transfer equilibrium in water and the corresponding expression for K a and (b) The chemical equation for the proton transfer equilibrium of the...
-
Calculate the pH of 0.15 m H 2 SO 4 (aq) at 25C.
-
Air-track gliders with masses 300 g, 400 g, and 200 g are lined up and held in place with lightweight springs compressed between them. All three are released at once. The 200 g glider flies off to...
-
Pulleys C and D in Figure are fastened together. Weights A and B are supported by ropes wound around the pulleys as shown. The radius for pulley C is 187 mm and the radius for pulley D is 138 mm. If...
-
As a leader what are some of the thoughtful and creative ideas that you have implemented to motivate your team and increase job satisfaction?
-
A Chinese smartphone maker TECNO Ltd has provided you with a summary of its price and cost information for one of its product segments (tablets). It is based on 2018 income statement. Units produced...
-
The following projected financial data is available for the single product of Janis Ltd:- October November December Sales (unit) 50,000 65,000 65,000 Production (unit) 70,000 60,000 50,000 Opening...
-
I would appreciate freehand sketches for the top, side views, and front views. D C 6 50 B 2.75 A a 5 4 3 2 1 .45 UNLESS OTHERWISE SPECIFIED: DIMENSIONS ARE IN MILLIMETERS SURFACE FINISH: TOLERANCES:...
-
A researcher applied the carcinogenic (cancer-causing) compound benzo (a) pyrene to the skin of five mice, and measured the concentration in the liver tissue after 48 hours. The results (nmol/gm)...
-
Find the inverse, if it exists, for the matrix. -1
-
Explain how a quantum dot can absorb light over a range of wavelengths and emit light over a much smaller range of wavelengths.
-
Explain why the speed of the particle needs to be taken into account in calculating the probability for transmission over a step potential.
-
The reflection probability from a step potential was calculated for E > V 0 in Section 16.5. Is Equation (16.18) valid for E < V 0 ? What information can you extract from Figure 16.1 that will allow...
-
Ellis Perry is an electronics components manufacturer. Information about the company's two products follows: \ table [ [ , , , ] , [ Units produced,AM - 2 , FM - 9 , ] , [ Direct labor hours required...
-
Which of the following requirements to claim Earned Income Tax Credit is TRUE? The credit can be claimed under any filing status. The taxpayer must have a valid SSN for employment in the U.S., issued...
-
Olde Tyme Beverage Companys operating activities for the year are listed below. Cost of Goods Manufactured $131,000 Operating expenses 80,000 Beginning inventory, FG 16,000 Ending inventory, FG...

Study smarter with the SolutionInn App


