Below are ball-and-stick models of two molecules. In each case, indicate whether there must be, may be,
Question:
Below are ball-and-stick models of two molecules. In each case, indicate whether there must be, may be, or cannot be one or more lone pairs of electrons on the central atom.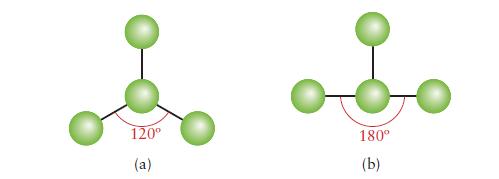
Transcribed Image Text:
120° (a) 180° (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
X 120 each M X b Explanation a structure where the central at...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Below are ball-and-stick models of two molecules. In each case, indicate whether there must be, may be, or cannot be one or more lone pairs of electrons on the central atom. 120 (a) 180 (b)
-
What is the amount of a loss that an insured person agrees to bear personally? _______ is the amount of a loss that an insured person agrees to bear personally. What is the effect in the auto...
-
Draw the Lewis structure for SeS 3 and answer the following questions. How many valence electrons are present in this compound? How many bonding electrons are present in this compound? How many lone...
-
What conditions must be met for revenue to be recorded? Can pledges meet those conditions?
-
Jewett Online Company has the following liability accounts after posting adjusting entries: Accounts Payable $63,000, Unearned Ticket Revenue $24,000, Estimated Warranty Liability $18,000, Interest...
-
Let y 1 , y 2 , . . . , y n be a random sample from a normal distribution, with mean and variance 2 . Show that the variance of the sampling distribution of s 2 is 2 4 /(n - 1).
-
What is Project Human Resources Management? AppendixLO1
-
Butler Manufacturing Corporation raised capital for a plant expansion by borrowing from a bank and making a stock offering. Butler engaged Weaver, CPA, to audit its December 2014 financial...
-
The percentsoe change in bond A ia Bound to tho decinal paces)
-
The sulfate ion, SO 4 2 , is present in a number of important minerals, including gypsum (CaSO 4 2H 2 O), which is used in cement, and Epsom salts (MgSO 4 7H 2 O), which is used as a purgative....
-
Draw the Lewis structure of (a) CCl 4 ; (b) COCl 2 ; (c) ONF; (d) NF 3 .
-
The following facts apply to Walken Company during December 2018: a. Walken began December with an accounts receivable balance (net of bad debts) of 25,000. b. Walken had credit sales of 85,000. c....
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
Describe the straight piecework plan, the 100-percent bonus plan, and the group bonus plan.
-
1. Firms may hold financial assets to earn returns. How the firm would classify financial assets? What treatment will such financial assets get in the financial statements in accordance with US GAAP...
-
A 230.-mL sample of a 0.275 M CaCl 2 solution is left on a hot plate overnight; the following morning, the solution is 1.10 M. What volume of water evaporated from the 0.275 M CaCl 2 solution?
-
A 50.00-mL sample of a solution containing Fe 2+ ions is titrated with a 0.0216 M KMnO 4 solution. It required 20.62 mL of KMnO4 solution to oxidize all the Fe 3+ ions to Fe 3+ ions by the reaction
-
A 50.00-mL sample of a solution containing Fe 2+ ions is titrated with a 0.0216 M KMnO 4 solution. It required 20.62 mL of KMnO4 solution to oxidize all the Fe 3+ ions to Fe 3+ ions by the reaction
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


