Consider atoms with the following electron configurations: Which atom has the largest first ionization energy, and which
Question:
Consider atoms with the following electron configurations:
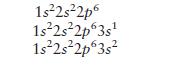
Which atom has the largest first ionization energy, and which has the smallest second ionization energy? Explain your choices.
Transcribed Image Text:
1s2s2p6 1s2s2p63s 1s2s2p 3s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The atom with the largest value of I is the one with the configu ration 1s2...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Graph f(x) - x, g(x) x +3 and (x) x5. Calculate the derivatives of f, g and h.
-
Identify the outer electron configurations for the (a) alkali metals, (b) alkaline earth metals, (c) halogens, (d) noble gases. For each electronic configuration given, choose the electronic...
-
Consider the formation of atomic hydrogen in the reaction c + H+ = H, where e is an electron, as the adsorption of an electron on a proton H+. (a) Show that the equilibrium concentrations of the...
-
Consider a situation with J identical firms that have marginal abatement cost functions for j=1,,J. The marginal damage function is equal to D'(E)=d.EDetermine the optimal allocation and the optimal...
-
Where g is the gravitational field strength, determine the value of T. T = 27V V/gA
-
Dean's Furniture Company assembles regular and deluxe kitchen cabinets from precut lumber. The regular cabinets are painted white, and the deluxe are varnished. Both painting and varnishing are...
-
1 Consider the implications of differences on Hofstedes first two dimensions of culture for management in the countries concerned. For example, what would Hofstedes conclusions lead you to predict...
-
The Plant Department of the local telephone company purchased four special pole hole diggers 8 years ago for $14,000 each. They have been in constant use to the present. Owing to an increased...
-
On December 30, 2020, Mr. and Mrs. Anchors personally owned yacht was wrecked in a federally declared disaster. Based on the following information, what is the amount of loss Mr. and Mrs. Anchor can...
-
Predict the trend in radius of the following ions: Be 2+ , Mg 2+ , Ca 2+ , and Sr 2+ .
-
The first ionization energy for phosphorus is 1060 kJ/mol, and that for sulfur is 1005 kJ/mol. Why?
-
Boardwalk Web Design has just completed operations for the year ended December 31, 2025. This is the third year of operations for the company. The following data have been assembled for the business:...
-
Perhaps we need a way to differentiate ourselves from the competition? Is it possible that we are dividing the customer's time too much? Does this mean that we should instead look to attract more...
-
Complete these answers with full paragraph sentences. 1)What are the Mission, Vision, & Values of the Palo Alto Network? 2) What are the Four Functions of Management Planning, Organizing, Leading, &...
-
One highly visible trait of a successful leader is that of role model: behavior exhibited by a leader is carefully observed and often sets the tone for the entire center. As a role model, it is...
-
Design a flowchart that illustrates the key processes and decision points within the custom leadership system, along with the various inputs and outputs. At the center of the flowchart is the leader,...
-
Prepare a sample memo to those that have been selected to serve on the "Bulletin 1" committee. Remind them of their charge and outline a calendar of meetings. Lastly, include a list of resources. ...
-
Determine the thermal efficiency of the regenerative Rankine cycle of Prob. 10-48 when the isentropic efficiency of the turbine before and after steam extraction point is 90 percent and the condenser...
-
Phosgene, COCl2, is a toxic gas used in the manufacture of urethane plastics. The gas dissociates at high temperature. At 400oC, the equilibrium constant Kc is 8.05 104. Find the percentage of...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: The anode of an...
-
(a) When the pH of 0.10 m HClO 2 (aq) was measured, it was found to be 1.2. What are the values of K a and pK a of chlorous acid? (b) The pH of a 0.10 m propylamine, C 3 H 7 NH 2 , aqueous solution...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Given the following financial data for the Smith Corporation, calculate the length of the firm's operating cycle (OC). Sales $2,610,000 Cost of Good Sold $2,088,000 Inventory $ 278,400 Accounts...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...

Study smarter with the SolutionInn App


